Abstract
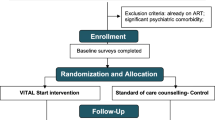
We developed and piloted a video-based intervention targeting HIV-positive pregnant women to optimize antiretroviral therapy (ART) retention and adherence by providing a VITAL Start (Video-intervention to Inspire Treatment Adherence for Life) before ART. VITAL Start (VS) was grounded in behavior-determinant models and developed through an iterative multi-stakeholder process. Of 306 pregnant women eligible for ART, 160 were randomized to standard of care (SOC), 146 to VS and followed for one-month. Of those assigned to VS, 100% completed video-viewing; 96.5% reported they would recommend VS. Of 11 health workers interviewed, 82% preferred VS over SOC; 91% found VS more time-efficient. Compared to SOC, VS group had greater change in HIV/ART knowledge (p < 0.01), trend towards being more likely to start ART (p = 0.07), and better self-reported adherence (p = 0.02). There were no significant group differences in 1-month retention and pharmacy pill count. VITAL Start was highly acceptable, feasible, with promising benefits to ART adherence.

Similar content being viewed by others
References
Joint United Nations Programme on HIV/AIDS. Ending AIDS: progress towards the 90–90–90 targets. Geneva: Joint United Nations Programme on HIV/AIDS; 2017.
World Health Organization. Global Health Observatory data respository. http://apps.who.int/gho/data/node.main.627?lang=en.
Joint United Nations Programme on HIV/AIDS. On the Fast-Track to an AIDS-free generation. Geneva: Joint United Nations Programme on HIV/AIDS; 2016.
Chersich MF, Newbatt E, Ng’oma K, de Zoysa I. UNICEF’s contribution to the adoption and implementation of option B+ for preventing mother-to-child transmission of HIV: a policy analysis. BMC Glob Health. 2018;14(1):55.
Adakun SA, Siedner MJ, Muzoora C, et al. Higher baseline CD4 cell count predicts treatment interruptions and persistent viremia in patients initiating ARVs in rural Uganda. J Acquir Immune Defic Syndr. 2013;62(3):317–21.
Haas AD, Msukwa MT, Egger M, et al. Adherence to antiretroviral therapy during and after pregnancy: cohort Study on women receiving care in Malawi’s Option B+ Program. Clin Infect Dis. 2016;63(9):1227–35.
The INSIGHT Start Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795–807.
Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505.
Tenthani L, Haas AD, Tweya H, et al. Retention in care under universal antiretroviral therapy for HIV infected pregnant and breastfeeding women (“Option B+”) in Malawi. AIDS. 2014;28(4):589–98.
Tweya H, Gugsa S, Hosseinipour M, et al. Understanding factors, outcomes and reasons for loss to follow-up among women in Option B+ PMTCT programme in Lilongwe, Malawi. Trop Med Int Health. 2014;19(11):1360–6.
Napua M, Pfeiffer JT, Chale F, et al. Option B+ in Mozambique: formative research findings for the design of a facility-level clustered randomized controlled trial to improve ART retention in antenatal care. J Acquir Immune Defic Syndr. 2016;72(Suppl 2):S181–8.
Puttkammer N, Domercant JW, Adler M, et al. ART attrition and risk factors among Option B+ patients in Haiti: a retrospective cohort study. PLoS ONE. 2017;12(3):e0173123.
Okawa S, Chirwa M, Ishikawa N, et al. Longitudinal adherence to antiretroviral drugs for preventing mother-to-child transmission of HIV in Zambia. BMC Pregn. Childbirth. 2015;15:258.
Llenas-Garcia J, Wikman-Jorgensen P, Hobbins M, et al. Retention in care of HIV-infected pregnant and lactating women starting ART under Option B+ in rural Mozambique. Trop Med Int Health. 2016;21(8):1003–12.
Dzangare J, Takarinda KC, Harries AD, et al. HIV testing uptake and retention in care of HIV-infected pregnant and breastfeeding women initiated on ‘Option B+ ’ in rural Zimbabwe. Trop Med Int Health. 2016;21(2):202–9.
Erlwanger AS, Joseph J, Gotora T, et al. Patterns of hiv care clinic attendance and adherence to antiretroviral therapy among pregnant and breastfeeding women living With HIV in the context of Option B+ in Zimbabwe. J Acquir Immune Defic Syndr. 2017;75(Suppl 2):S198–s206.
Clouse K, Schwartz S, Van Rie A, Bassett J, Yende N, Pettifor A. “What they wanted was to give birth; nothing else”: barriers to retention in option B+ HIV care among postpartum women in South Africa. J Acquir Immune Defic Syndr. 2014;67(1):e12–8.
Gugsa S, Potter K, Tweya H, et al. Exploring factors associated with ART adherence and retention in care under Option B+ strategy in Malawi: a qualitative study. PLoS ONE. 2017;12(6):e0179838.
Kim MH, Mazenga A, Simon K, Yu X, Ahmed S, Nyasulu P, et al. Burnout and self-reported suboptimal patient care amongst health care workers providing HIV care in Malawi. Hum Resour Health. 2017;13:e0192983.
Ahmed S, Kim MH, Abrams EJ. Risks and benefits of lifelong antiretroviral treatment for pregnant and breastfeeding women: a review of the evidence for the Option B+ approach. Curr Opin HIV AIDS. 2013;8(5):473–88.
Kim MH, Ahmed S, Buck WC, et al. The Tingathe programme: a pilot intervention using community health workers to create a continuum of care in the prevention of mother to child transmission of HIV (PMTCT) cascade of services in Malawi. J Int AIDS Soc. 2012;15(Suppl 2):17389.
Kanters S, Park JJ, Chan K, et al. Use of peers to improve adherence to antiretroviral therapy: a global network meta-analysis. J Int AIDS Soc. 2016;19(1):21141.
Sweat M, O’Donnell C, O’Donnell L. Cost-effectiveness of a brief video-based HIV intervention for African American and Latino sexually transmitted disease clinic clients. AIDS. 2001;15(6):781–7.
Chiasson MA, Shaw FS, Humberstone M, Hirshfield S, Hartel D. Increased HIV disclosure three months after an online video intervention for men who have sex with men (MSM). AIDS Care. 2009;21(9):1081–9.
Tuong W, Larsen ER, Armstrong AW. Videos to influence: a systematic review of effectiveness of video-based education in modifying health behaviors. J Behav Med. 2014;37(2):218–33.
Wong IY, Lawrence NV, Struthers H, McIntyre J, Friedland GH. Development and assessment of an innovative culturally sensitive educational videotape to improve adherence to highly active antiretroviral therapy in Soweto, South Africa. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S142–8.
Fisher HH, Patel-Larson A, Green K, et al. Evaluation of an HIV prevention intervention for African Americans and Hispanics: findings from the VOICES/VOCES Community-based Organization Behavioral Outcomes Project. AIDS Behav. 2011;15(8):1691–706.
Neumann MS, O’Donnell L, Doval AS, et al. Effectiveness of the VOICES/VOCES sexually transmitted disease/human immunodeficiency virus prevention intervention when administered by health department staff: does it work in the “real world”? Sex Transm Dis. 2011;38(2):133–9.
Sobel RM, Paasche-Orlow MK, Waite KR, Rittner SS, Wilson EA, Wolf MS. Asthma 1-2-3: a low literacy multimedia tool to educate African American adults about asthma. J Commun Health. 2009;34(4):321–7.
Israel BA, Schulz AJ, Parker EA, Becker AB. Community-based participatory research: policy recommendations for promoting a partnership approach in health research. Educ Health (Abingdon, England). 2001;14(2):182–97.
Rhodes SD, Hergenrather KC, Montano J, et al. Using community-based participatory research to develop an intervention to reduce HIV and STD infections among Latino men. IADS Educ Prev. 2006;18(5):375–89.
Bandura A. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs: Prentice-Hall; 1986.
Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process. 1991;50(2):179–211.
Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychol. 2006;25(4):462–73.
Jensen JD. The relative persuasiveness of gain-framed loss-framed messages for encouraging disease prevention behaviors: a meta-analytic review AU - O’Keefe, Daniel J. J Health Commun. 2007;12(7):623–44.
Kim MH, Zhou A, Mazenga A, et al. Why Did I Stop? Barriers and Facilitators to Uptake and Adherence to ART in Option B+ HIV Care in Lilongwe, Malawi. PLoS ONE. 2016;11(2):e0149527.
The World Bank. World Bank Online Data. 2018.
ICAP. Malawi Population-Based HIV Impact Assessment: MPHIA 2015–2016.; 2016.
National Statistical Office Malawi, ICF International. Malawi Demographic and Health Survey 2015-16: Key Indicators Report. Zomba, Malawi; 2016.
Malawi Ministry of Health. Clinical Management of HIV in Children and Adults. 3rd ed. Lilongwe: Malawi Ministry of Health; 2016.
World Health Organizaion. A User’s Guide to the Self Reporting Questionnaire (SRQ)1994. https://apps.who.int/iris/handle/10665/61113.
Zimet GD, Dahlem NW, Zimet SG, Farley GK. The Multidimensional Scale of Perceived Social Support Journal of Personality Assessment. 1988;52(1):30–41.
World Health Organization. WHO Violence Against Women Instrument. 2003.
Saunder JB, Aasland OG, Babor TF, De La Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction. 1993;88(6):791–804.
Berman AH, Bergman H, Palmstierna T, Schlyter F. Evaluation of the Drug Use Disorders Identification Test (DUDIT) in criminal justice and detoxification settings and in a Swedish population sample. Eur Addict Res. 2005;11(1):22–31.
Stewart RC, Kauye F, Umar E, et al. Validation of a Chichewa version of the self-reporting questionnaire (SRQ) as a brief screening measure for maternal depressive disorder in Malawi, Africa. J Affect Disord. 2009;112(1–3):126–34.
Stewart RC, Umar E, Tomenson B, Creed F. Validation of the multi-dimensional scale of perceived social support (MSPSS) and the relationship between social support, intimate partner violence and antenatal depression in Malawi. BMC Psychiatry. 2014;14:180.
Seth P, Glenshaw M, Sabatier JHF, et al. AUDIT, AUDIT-C, and AUDIT-3: drinking Patterns and Screening for Harmful, Hazardous and Dependent Drinking in Katutura, Namibia. PLoS ONE. 2015;10(3):e0120850.
Berman AH BH PT, Schlyter F. The Drug Use Disorders Identification Test (DUDIT) Manual. 1.0 ed. Stockholm: Karolinska Institutet, Department of Clinical Neuroscience, Section for Alcohol and Drug Dependence Research; 2003.
Love EM, Manalo IF, Chen SC, Chen KH, Stoff BK. A video-based educational pilot for basal cell carcinoma (BCC) treatment: a randomized controlled trial. J Am Acad Dermatol. 2016;74(3):477–83.e7.
Myer L, Phillips TK, Zerbe A, et al. Optimizing antiretroviral therapy (ART) for maternal and child health (MCH): rationale and design of the MCH-ART Study. J Acquir Immune Defic Syndr. 2016;72(Suppl 2):S189–96.
Johnson MO, Neilands TB, Dilworth SE, Morin SF, Remien RH, Chesney MA. The role of self-efficacy in HIV treatment adherence: validation of the HIV Treatment Adherence Self-Efficacy Scale (HIV-ASES). J Behav Med. 2007;30(5):359–70.
Phillips T, Brittain K, Mellins CA, et al. A self-reported adherence measure to screen for elevated HIV viral load in pregnant and postpartum women on antiretroviral therapy. AIDS Behav. 2017;21(2):450–61.
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline: Statistical Principles for Clinical Trials E9. ICH Harmonised Tripartite Guideline: Statistical Principles for Clinical Trials. European Medicines Agency. 1998.
Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40(7–8):1280–8.
Ortego C, Huedo-Medina TB, Llorca J, et al. Adherence to highly active antiretroviral therapy (HAART): a meta-analysis. AIDS Behav. 2011;15(7):1381–96.
Acknowledgements
We thank the Ministry of Health for their partnership in this endeavor. We are very grateful to the women living with HIV, families, community members, and HCWs who participated in the development and piloting of the VS intervention. We appreciate the support provided by all VITAL Start team members.
Disclaimer
The contents of this report are the sole responsibility of the authors and do not necessarily reflect the views of the National Institute for Health or the United States Government.
Funding
Support for this study was made possible by the Fogarty International Center of the National Institutes of Health under Award Number K01 TW009644 as well the National Institute of Mental Health R01MH115793.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, M.H., Ahmed, S., Tembo, T. et al. VITAL Start: Video-Based Intervention to Inspire Treatment Adherence for Life—Pilot of a Novel Video-Based Approach to HIV Counseling for Pregnant Women Living with HIV. AIDS Behav 23, 3140–3151 (2019). https://doi.org/10.1007/s10461-019-02634-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-019-02634-1