Abstract
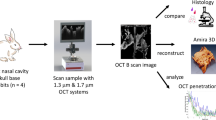
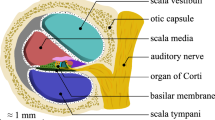
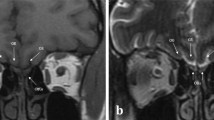
Inspecting the olfactory cleft can be of high interest, as it is an open access to neurons, and thus an opportunity to collect in situ related data in a non-invasive way. Also, recent studies show a strong link between olfactory deficiency and neurodegenerative diseases such as Alzheimer and Parkinson diseases. However, no inspection of this area is possible today, as it is very difficult to access. Only robot-assisted interventions seem viable to provide the required dexterity. The feasibility of this approach is demonstrated in this article, which shows that the path complexity to the olfactory cleft can be managed with a concentric tube robot (CTR), a particular type of continuum robot. First, new anatomical data are elaborated, in particular for the olfactory cleft, that remains hardly characterized. 3D reconstructions are conducted on the database of 20 subjects, using CT scan images. Measurements are performed to describe the anatomy, including metrics with inter-subject variability. Then, the existence of collision-free passageways for CTR is shown using the 3D reconstructions. Among the 20 subjects, 19 can be inspected using only 3 different robot geometries. This constitutes an essential step towards a robotic device to inspect subjects for clinical purposes.








Similar content being viewed by others
References
Aarli, J. A., T. Dua, A. Janca, and A. Muscetta. Neurological Disorders: Public Health Challenges. Geneva: World Health Organization, 2006, pp. 32.
Amorim, P., T. Moraes, J. Silva, and H. Pedrini. InVesalius: An interactive rendering framework for health care support. In: Advances in Visual Computing, 2015, vol. 9474, pp. 45–54.
AGILTRON. Miniature oct fiber probe. http://www.agiltron.com/PDFs/Miniature
Bergeles, C., A. H. Gosline, N. V. Vasilyev, P. J. Codd, P. J. del Nido, and P. E. Dupont. Concentric tube robot design and optimization based on task and anatomical constraints. IEEE Trans. Robot. 31(1):67–84, 2015.
Bojsen-Moller, F. and J. Fahrenkrug. Nasal swell-bodies and cyclic changes in the air passage of the rat and rabbit nose. J. Anat. 110:25, 1971.
Bruyas, A., F. Geiskopf, and P. Renaud. Toward unibody robotic structures with integrated functions using multimaterial additive manufacturing: case study of an MRI-compatible interventional device. In: IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), 2015, pp.1744–1750.
Bui, N. L., S. H. Ong, and K. W. C. Foong. Automatic segmentation of the nasal cavity and paranasal sinuses from cone-beam CT images.Int. J. Comput. Assist. Radiol. Surg. 10(8):1269–1277, 2015.
Burgner-Kahrs, J., D. C. Rucker, and H. Choset. Continuum robots for medical applications: a survey. IEEE Trans. Robot. 31(6):1261–1280, 2015.
Burgner, J., P. J. Swaney, R. Lathrop, K. D. Weaver, R. J. Webster et al. Debulking from within: a robotic steerable cannula for intracerebral hemorrhage evacuation. IEEE Trans. Biomed. Eng. 60(9):2567–2575, 2013.
Butler, E. J., R. Hammond-Oakley, S. Chawarski, A. H. Gosline, P. Codd, T. Anor, J. R. Madsen, P. E. Dupont, and J. Lock. Robotic neuro-endoscope with concentric tube augmentation. In: IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), 2012, pp.2941–2946.
Cignoni, P., M. Callieri, M. Corsini, M. Dellepiane, F. Ganovelli, and G. Ranzuglia. MeshLab: an open-source mesh processing tool. In: Sixth Eurographics Italian Chapter Conference, 2008, pp.129–136.
Costanzo, R. M. Regeneration of olfactory receptor cells. Ciba Found. Symp. 160:233–248, 1991.
Doty, R. L. Olfactory dysfunction in Parkinson disease. Nat. Rev. Neurol. 8(6):329–339, 2012.
Dryer, L. and P. Graziadei. Influence of the olfactory organ on brain development. Perspect. Dev. Neurobiol. 2(2):163–174, 1994.
Dupont, P. E., J. Lock, B. Itkowitz, and E. Butler. Design and control of concentric-tube robots. IEEE Trans. Robot. 26(2):209–225, 2010.
Elwany, S., A. Medanni, M. Eid, A. Aly, A. El-Daly, and S. Ammar. Radiological observations on the olfactory fossa and ethmoid roof. J. Laryngol. Otol. 124(12):1251–1256, 2010.
Escada, P. A., C. Lima, and J. M. da Silva. The human olfactory mucosa. Eur. Arch. Oto-Rhino-Laryngol. 124(12):1251–1256, 2010.
Flood, D. G. and P. D. Coleman. Neuron numbers and sizes in aging brain: comparisons of human, monkey, and rodent data. Neurobiol. Aging 9:453–463, 1988.
Gilbert, H. B., J. Neimat, and R. J. Webster. Concentric tube robots as steerable needles: achieving follow-the-leader deployment. IEEE Trans. Robot. 31(2):246–258, 2015.
Gladwin, K. and D. Choi. Olfactory ensheathing cells: part Icurrent concepts and experimental laboratory models. World Neurosurg. 83(1):114–119, 2015.
Godoy, M. D. C. L., R. L. Voegels, F. de Rezende Pinna, R. Imamura, and J. M. Farfel. Olfaction in neurologic and neurodegenerative diseases: a literature review. Int. Arch. Otorhinolaryngol. 19(2):176–179, 2015.
Gopinath, B., K. J. Anstey, A. Kifley, and P. Mitchell. Olfactory impairment is associated with functional disability and reduced independence among older adults. Maturitas 72(1):50–55, 2012.
Gosline, A. H., N. V. Vasilyev, E. J. Butler, C. Folk, A. Cohen, R. Chen, N. Lang, P. J. Del Nido, and P. E. Dupont. Percutaneous intracardiac beating-heart surgery using metal mems tissue approximation tools. Int. J. Robot. Res. 31(9):1081–1093, 2012.
Hudson, T. C., M. C. Lin, J. Cohen, S. Gottschalk, and D. Manocha. V-COLLIDE: accelerated collision detection for VRML. In: Proceedings of the second symposium on Virtual reality modeling language, 1997, pp.117–123.
Jones, B. A. and I. D. Walker. Kinematics for multisection continuum robots. IEEE Trans. Robot. 22(1):43–55, 2006.
Kalmey, J. K., J. Thewissen, and D. E. Dluzen. Age-related size reduction of foramina in the cribriform plate. Anat. Record 251(3):326–329, 1998.
Kavoi, B. M. and H. Jameela. Comparative morphometry of the olfactory bulb, tract and stria in the human, dog and goat. Int. J. Morphol. 29(3):939–946, 2011.
Lavoie, J., P. Gass Astorga, H. Segal-Gavish, Y. Wu, Y. Chung, N. Cascella, A. Sawa, and K. Ishizuka. The olfactory neural epithelium as a tool in neuroscience. Trends Mol. Med. 23(2):100–103, 2017.
Lorensen, W. E. and H. E. Cline. Marching cubes: a high resolution 3D surface construction algorithm. SIGGRAPH Comput. Graph. 21(4):163–169, 1987.
Moench, T., R. Gasteiger, G. Janiga, H. Theisel, and B. Preim. Context-aware mesh smoothing for biomedical applications. Comput. Graph. 35(4):755–767, 2011.
Moon, C., S. J. Yoo, and H. S. Han. Smell, Encyclopedia of the Neurological Sciences, 2nd ed. Cambridge: Academic Press, 2014, pp. 216–220.
Renevier R., Tamadazte B., Rabenorosoa K., Tavernier L., and Andreff N. Endoscopic laser surgery: design, modeling and control. IEEE/ASME Trans. Mech. 22(1):99–106, 2017.
Robert J. Webster, I. and B. A. Jones. Design and kinematic modeling of constant curvature continuum robots: a review. Int. J. Robot. Res. 29(13):1661–1683, 2010.
Savvateeva, D. M., C. Güldner, T. Murthum, S. Bien, A. Teymoortash, J. A. Werner, and M. Bremke. Digital volume tomography (DVT) measurements of the olfactory cleft and olfactory fossa. Acta Oto-Laryngol. 130(3):398–404, 2010.
Acknowledgments
This work was supported by the French National Agency for Research within the Biomedical Innovation program (NEMRO ANR-14-CE17-0013), and the Investissements d’Avenir (Robotex ANR-10-EQPX-44, Labex CAMI ANR-11-LABX-0004 and Labex ACTION ANR-11-LABX-0001-01).
Conflict of interest
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Cameron N. Riviere oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Girerd, C., Lihoreau, T., Rabenorosoa, K. et al. In Vivo Inspection of the Olfactory Epithelium: Feasibility of Robotized Optical Biopsy. Ann Biomed Eng 46, 1951–1961 (2018). https://doi.org/10.1007/s10439-018-2076-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-018-2076-9