Abstract
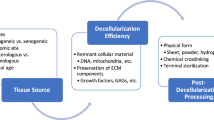
Successful regenerative medicine strategies for functional tissue reconstruction include the in situ placement of acellular materials composed of the extracellular matrix (ECM) or individual components of the ECM. The composition and ultrastructure of these materials vary depending on multiple factors including the tissue source and species from which the materials are harvested, the methods of manufacture, the efficiency of decellularization, post-processing modifications such as chemical cross-linking or solubilization, and the methods of terminal sterilization. Appropriately configured materials have the ability to modulate different stages of the healing response by inducing a shift from a process of inflammation and scar tissue formation to one of constructive remodeling and functional tissue restoration. The events that facilitate such a dramatic change during the biomaterial-host interaction are complex and necessarily involve both the immune system and mechanisms of stem cell recruitment, growth, and differentiation. The present manuscript reviews the composition of biologic scaffolds, the methods and recommendations for manufacture, the mechanisms of the biomaterial–host interaction, and the clinical application of this regenerative medicine approach.






Similar content being viewed by others
References
Agrawal, V., J. Kelly, S. Tottey, K. A. Daly, S. A. Johnson, B. F. Siu, J. Reing, and S. F. Badylak. An isolated cryptic peptide influences osteogenesis and bone remodeling in an adult mammalian model of digit amputation. Tissue Eng. Part A 17(23–24):3033–3044, 2011.
Aller, M. A., J. I. Arias, L. A. Arraez-Aybar, C. Gilsanz, and J. Arias. Wound healing reaction: a switch from gestation to senescence. World J. Exp. Med. 4(2):16–26, 2014.
Ambrosio, F., S. L. Wolf, A. Delitto, G. K. Fitzgerald, S. F. Badylak, M. L. Boninger, and A. J. Russell. The emerging relationship between regenerative medicine and physical therapeutics. Phys. Ther. 90(12):1807–1814, 2010.
Badylak, S. F. The extracellular matrix as a scaffold for tissue reconstruction. Semin. Cell Dev. Biol. 13(5):377–383, 2002.
Badylak, S. F. Decellularized allogeneic and xenogeneic tissue as a bioscaffold for regenerative medicine: factors that influence the host response. Ann. Biomed. Eng. 42(7):1517–1527, 2014.
Badylak, S. F., D. O. Freytes, and T. W. Gilbert. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 5(1):1–13, 2009.
Badylak, S. F., T. Hoppo, A. Nieponice, T. W. Gilbert, J. M. Davison, and B. A. Jobe. Esophageal preservation in five male patients after endoscopic inner-layer circumferential resection in the setting of superficial cancer: a regenerative medicine approach with a biologic scaffold. Tissue Eng. Part A 17(11–12):1643–1650, 2011.
Badylak, S. F., D. A. Vorp, A. R. Spievack, A. Simmons-Byrd, J. Hanke, D. O. Freytes, A. Thapa, T. W. Gilbert, and A. Nieponice. Esophageal reconstruction with ECM and muscle tissue in a dog model. J. Surg. Res. 128(1):87–97, 2005.
Baker, S. M., R. V. Sugars, M. Wendel, A. J. Smith, R. J. Waddington, P. R. Cooper, and A. J. Sloan. TGF-beta/extracellular matrix interactions in dentin matrix: a role in regulating sequestration and protection of bioactivity. Calcif. Tissue Int. 85(1):66–74, 2009.
Birch, H. L., C. T. Thorpe, and A. P. Rumian. Specialisation of extracellular matrix for function in tendons and ligaments. Muscles Ligaments Tendons J. 3(1):12–22, 2013.
Bissell, M. J., and J. Aggeler. Dynamic reciprocity: how do extracellular matrix and hormones direct gene expression? Prog. Clin. Biol. Res. 249:251–262, 1987.
Brennan, E. P., J. Reing, D. Chew, J. M. Myers-Irvin, E. J. Young, and S. F. Badylak. Antibacterial activity within degradation products of biological scaffolds composed of extracellular matrix. Tissue Eng. 12(10):2949–2955, 2006.
Brown, B. N., R. Londono, S. Tottey, L. Zhang, K. A. Kukla, M. T. Wolf, K. A. Daly, J. E. Reing, and S. F. Badylak. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 8(3):978–987, 2012.
Brown, B. N., B. D. Ratner, S. B. Goodman, S. Amar, and S. F. Badylak. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 33(15):3792–3802, 2012.
Brown, B. N., J. E. Valentin, A. M. Stewart-Akers, G. P. McCabe, and S. F. Badylak. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 30(8):1482–1491, 2009.
Butterfield, J. L. 440 consecutive immediate, implant-based, single-surgeon breast reconstructions in 281 patients: a comparison of early outcomes and costs between surgimend fetal bovine and alloderm human cadaveric acellular dermal matrices. Plast. Reconstr. Surg. 131(5):940–951, 2013.
Carey, L. E., C. L. Dearth, S. A. Johnson, R. Londono, C. J. Medberry, K. A. Daly, and S. F. Badylak. In vivo degradation of 14c-labeled porcine dermis biologic scaffold. Biomaterials 35(29):8297–8304, 2014.
Chattopadhyay, S., and R. T. Raines. Review collagen-based biomaterials for wound healing. Biopolymers 101(8):821–833, 2014.
Chen, M., M. P. Marinkovich, A. Veis, X. Cai, C. N. Rao, E. A. O’Toole, and D. T. Woodley. Interactions of the amino-terminal noncollagenous (NC1) domain of Type VII collagen with extracellular matrix components. A potential role in epidermal-dermal adherence in human skin. J. Biol. Chem. 272(23):14516–14522, 1997.
Clark, R. A., N. E. Wikner, D. E. Doherty, and D. A. Norris. Cryptic chemotactic activity of fibronectin for human monocytes resides in the 120-kDa fibroblastic cell-binding fragment. J. Biol. Chem. 263(24):12115–12123, 1988.
Crapo, P. M., T. W. Gilbert, and S. F. Badylak. An overview of tissue and whole organ decellularization processes. Biomaterials 32(12):3233–3243, 2011.
Crapo, P. M., C. J. Medberry, J. E. Reing, S. Tottey, Y. van der Merwe, K. E. Jones, and S. F. Badylak. Biologic scaffolds composed of central nervous system extracellular matrix. Biomaterials 33(13):3539–3547, 2012.
Daly, K. A., et al. The host response to endotoxin-contaminated dermal matrix. Tissue Eng. Part A 18(11–12):1293–1303, 2012.
Davis, G. E., K. J. Bayless, M. J. Davis, and G. A. Meininger. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am. J. Pathol. 156(5):1489–1498, 2000.
DiPietro, L. A. Angiogenesis and scar formation in healing wounds. Curr. Opin. Rheumatol. 25(1):87–91, 2013.
Giannelli, G., J. Falk-Marzillier, O. Schiraldi, W. G. Stetler-Stevenson, and V. Quaranta. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science 277(5323):225–228, 1997.
Gilbert, T. W., A. M. Stewart-Akers, A. Simmons-Byrd, and S. F. Badylak. Degradation and remodeling of small intestinal submucosa in canine achilles tendon repair. J. Bone Joint Surg. Am. 89(3):621–630, 2007.
Groulx, J. F., D. Gagne, Y. D. Benoit, D. Martel, N. Basora, and J. F. Beaulieu. Collagen VI is a basement membrane component that regulates epithelial cell–fibronectin interactions. Matrix Biol. 30(3):195–206, 2011.
Hodde, J. P., S. F. Badylak, A. O. Brightman, and S. L. Voytik-Harbin. Glycosaminoglycan content of small intestinal submucosa: a bioscaffold for tissue replacement. Tissue Eng. 2(3):209–217, 1996.
Hodde, J., R. Record, R. Tullius, and S. Badylak. Fibronectin peptides mediate HMEC adhesion to porcine-derived extracellular matrix. Biomaterials 23(8):1841–1848, 2002.
Hynes, R. O. The extracellular matrix: not just pretty fibrils. Science 326(5957):1216–1219, 2009.
Iozzo, R. V., I. R. Cohen, S. Grassel, and A. D. Murdoch. The biology of perlecan: the multifaceted heparan sulphate proteoglycan of basement membranes and pericellular matrices. Biochem. J. 302(Pt 3):625–639, 1994.
Iozzo, R. V., and A. D. Murdoch. Proteoglycans of the extracellular environment: clues from the gene and protein side offer novel perspectives in molecular diversity and function. FASEB J. 10(5):598–614, 1996.
Johnson, T. D., and K. L. Christman. Injectable hydrogel therapies and their delivery strategies for treating myocardial infarction. Expert Opin. Drug Deliv. 10(1):59–72, 2013.
Johnson, T. D., J. A. Dequach, R. Gaetani, J. Ungerleider, D. Elhag, V. Nigam, A. Behfar, and K. L. Christman. Human versus porcine tissue sourcing for an injectable myocardial matrix hydrogel. Biomater. Sci. 2014:60283D, 2014.
Keane, T. J., R. Londono, R. M. Carey, C. A. Carruthers, J. E. Reing, C. L. Dearth, A. D’Amore, C. J. Medberry, and S. F. Badylak. Preparation and characterization of a biologic scaffold from esophageal mucosa. Biomaterials 34(28):6729–6737, 2013.
Keane, T. J., R. Londono, N. J. Turner, and S. F. Badylak. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 33(6):1771–1781, 2012.
Kissane, N. A., and K. M. Itani. A decade of ventral incisional hernia repairs with biologic acellular dermal matrix: what have we learned? Plast. Reconstr. Surg. 130(5 Suppl 2):194S–202S, 2012.
Korpos, E., C. Wu, J. Song, R. Hallmann, and L. Sorokin. Role of the extracellular matrix in lymphocyte migration. Cell Tissue Res. 339(1):47–57, 2010.
Ladowski, J. M., and J. S. Ladowski. Retrospective analysis of bovine pericardium (vascu-guard) for patch closure in carotid endarterectomies. Ann. Vasc. Surg. 25(5):646–650, 2011.
Mantovani, A., A. Sica, S. Sozzani, P. Allavena, A. Vecchi, and M. Locati. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25(12):677–686, 2004.
Maxson, S., E. A. Lopez, D. Yoo, A. Danilkovitch-Miagkova, and M. A. Leroux. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl. Med. 1(2):142–149, 2012.
Medberry, C. J., et al. Hydrogels derived from central nervous system extracellular matrix. Biomaterials 34(4):1033–1040, 2013.
Mills, S. J., A. J. Cowin, and P. Kaur. Pericytes, mesenchymal stem cells and the wound healing process. Cells 2(3):621–634, 2013.
Mills, C. D., K. Kincaid, J. M. Alt, M. J. Heilman, and A. M. Hill. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 164(12):6166–6173, 2000.
Mogford, J. E., G. E. Davis, S. H. Platts, and G. A. Meininger. Vascular smooth muscle alpha v beta 3 integrin mediates arteriolar vasodilation in response to RGD peptides. Circ. Res. 79(4):821–826, 1996.
Mosser, D. M. The many faces of macrophage activation. J. Leukoc. Biol. 73(2):209–212, 2003.
Nauseef, W. M., and N. Borregaard. Neutrophils at work. Nat. Immunol. 15(7):602–611, 2014.
Nieponice, A., F. F. Ciotola, F. Nachman, B. A. Jobe, T. Hoppo, R. Londono, S. Badylak, and A. E. Badaloni. Patch esophagoplasty: esophageal reconstruction using biologic scaffolds. Ann. Thorac. Surg. 97(1):283–288, 2014.
Nieponice, A., T. W. Gilbert, S. A. Johnson, N. J. Turner, and S. F. Badylak. Bone marrow-derived cells participate in the long-term remodeling in a mouse model of esophageal reconstruction. J. Surg. Res. 182(1):e1–e7, 2013.
Ponce, M. L., M. Nomizu, M. C. Delgado, Y. Kuratomi, M. P. Hoffman, S. Powell, Y. Yamada, H. K. Kleinman, and K. M. Malinda. Identification of endothelial cell binding sites on the laminin gamma 1 chain. Circ. Res. 84(6):688–694, 1999.
Record, R. D., D. Hillegonds, C. Simmons, R. Tullius, F. A. Rickey, D. Elmore, and S. F. Badylak. In vivo degradation of 14C-labeled small intestinal submucosa (SIS) when used for urinary bladder repair. Biomaterials 22(19):2653–2659, 2001.
Reing, J. E., et al. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng. Part A 15(3):605–614, 2009.
Ricard-Blum, S., and L. Ballut. Matricryptins derived from collagens and proteoglycans. Front Biosci. (Landmark Ed) 16:674–697, 2011.
Rider, P., Y. Carmi, O. Guttman, A. Braiman, I. Cohen, E. Voronov, M. R. White, C. A. Dinarello, and R. N. Apte. IL-1alpha and IL-1beta recruit different myeloid cells and promote different stages of sterile inflammation. J. Immunol. 187(9):4835–4843, 2011.
Ruoslahti, E., E. G. Hayman, M. Pierschbacher, and E. Engvall, Fibronectin. Purification, immunochemical properties, and biological activities. Methods Enzymol. 82 Pt A:803–831, 1982.
Sarikaya, A., R. Record, C. C. Wu, B. Tullius, S. Badylak, and M. Ladisch. Antimicrobial activity associated with extracellular matrices. Tissue Eng. 8(1):63–71, 2002.
Sawkins, M. J., et al. Hydrogels derived from demineralized and decellularized bone extracellular matrix. Acta Biomater. 9(8):7865–7873, 2013.
Scholl, F. G., M. M. Boucek, K. C. Chan, L. Valdes-Cruz, and R. Perryman. Preliminary experience with cardiac reconstruction using decellularized porcine extracellular matrix scaffold: human applications in congenital heart disease. World J. Pediatr. Congenit. Heart Surg. 1(1):132–136, 2010.
Schonherr, E., M. Broszat, E. Brandan, P. Bruckner, and H. Kresse. Decorin core protein fragment Leu155-Val260 interacts with TGF-beta but does not compete for decorin binding to Type I collagen. Arch. Biochem. Biophys. 355(2):241–248, 1998.
Schonherr, E., H. Hausser, L. Beavan, and H. Kresse. Decorin-Type I collagen interaction. Presence of separate core protein-binding domains. J. Biol. Chem. 270(15):8877–8883, 1995.
Schwarzbauer, J. E., and J. L. Sechler. Fibronectin fibrillogenesis: a paradigm for extracellular matrix assembly. Curr. Opin. Cell Biol. 11(5):622–627, 1999.
Seiffert, D., and J. W. Smith. The cell adhesion domain in plasma vitronectin is cryptic. J. Biol. Chem. 272(21):13705–13710, 1997.
Seif-Naraghi, S. B., et al. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci. Transl. Med. 5(173):173ra25, 2013.
Sicari, B. M., et al. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci. Transl. Med. 6(234):234ra58, 2014.
Sicari, B. M., et al. The effect of source animal age upon the in vivo remodeling characteristics of an extracellular matrix scaffold. Biomaterials 33(22):5524–5533, 2012.
Slack, S. M., J. L. Bohnert, and T. A. Horbett. The effects of surface chemistry and coagulation factors on fibrinogen adsorption from plasma. Ann. N. Y. Acad. Sci. 516:223–243, 1987.
Smaniotto, S., D. A. Mendes-da-Cruz, C. E. Carvalho-Pinto, L. M. Araujo, M. Dardenne, and W. Savino. Combined role of extracellular matrix and chemokines on peripheral lymphocyte migration in growth hormone transgenic mice. Brain Behav. Immun. 24(3):451–461, 2010.
Soto-Gutierrez, A., et al. A whole-organ regenerative medicine approach for liver replacement. Tissue Eng. Part C 17(6):677–686, 2011.
Tran Cao, H. S., C. Tokin, J. Konop, H. Ojeda-Fournier, J. Chao, and S. L. Blair. A preliminary report on the clinical experience with alloderm in breast reconstruction and its radiologic appearance. Am. Surg. 76(10):1123–1126, 2010.
Turner, M. D., B. Nedjai, T. Hurst, and D. J. Pennington. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014.
Valentin, J. E., A. M. Stewart-Akers, T. W. Gilbert, and S. F. Badylak. Macrophage participation in the degradation and remodeling of extracellular matrix scaffolds. Tissue Eng. Part A 15(7):1687–1694, 2009.
van der Rest, M., and R. Garrone. Collagen family of proteins. FASEB J. 5(13):2814–2823, 1991.
Vorotnikova, E., et al. Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol. 29(8):690–700, 2010.
Wang, J., and H. Arase. Regulation of immune responses by neutrophils. Ann. N. Y. Acad. Sci. 1319(1):66–81, 2014.
Whitelock, J. M., A. D. Murdoch, R. V. Iozzo, and P. A. Underwood. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J. Biol. Chem. 271(17):10079–10086, 1996.
Wolf, M. T., K. A. Daly, E. P. Brennan-Pierce, S. A. Johnson, C. A. Carruthers, A. D’Amore, S. P. Nagarkar, S. S. Velankar, and S. F. Badylak. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials 33(29):7028–7038, 2012.
Wolf, M. T., K. A. Daly, J. E. Reing, and S. F. Badylak. Biologic scaffold composed of skeletal muscle extracellular matrix. Biomaterials 33(10):2916–2925, 2012.
Yamaguchi, Y., D. M. Mann, and E. Ruoslahti. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature 346(6281):281–284, 1990.
Zamarron, C., M. H. Ginsberg, and E. F. Plow. Monoclonal antibodies specific for a conformationally altered state of fibrinogen. Thromb. Haemost. 64(1):41–46, 1990.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Nadya Lumelsky oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Londono, R., Badylak, S.F. Biologic Scaffolds for Regenerative Medicine: Mechanisms of In vivo Remodeling. Ann Biomed Eng 43, 577–592 (2015). https://doi.org/10.1007/s10439-014-1103-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-014-1103-8