Abstract
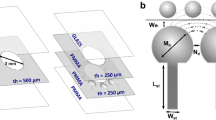
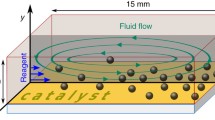
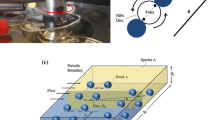
In this study, we developed a dynamic microfluidic device that enables the clustering of three different types of microbeads in a trapping spot and the rearrangement of contacting modes of the clustered microbeads. To achieve these two functions, two features are added to the conventional dynamic microfluidic device. (1) To trap multiple beads, an extended trapping spot with sub-by-pass channels and a valve was employed. (2) To rearrange the clustered microbeads, trapping spots that work only by backward flow were added. The design of the microfluidic device was realized by calculations based on fluidic resistance. By using the designed device, we successfully clustered different types of hydrogel microbeads including target materials, and observed reactions between clustered microbeads. In addition, by rearranging the contacting modes of the clustered microbeads, the reaction could be initiated/terminated at the desired time. We found that this dynamic microfluidic device is applicable to the quantitative analysis of chemical reactions between small amounts of multiple materials.






Similar content being viewed by others
References
Abbyad P, Dangla R, Alexandrou A, Baroud CN (2011) Rails and anchors: guiding and trapping droplet microreactors in two dimensions. Lab Chip 11:813–821
Amador C, Gavriilidis A, Angeli P (2004) Flow distribution in different microreactor scale-out geometries and the effect of manufacturing tolerances and channel blockage. Chem Eng J 101:379–390
Bai YP, He XM, Liu DS, Patil SN, Bratton D, Huebner A, Hollfelder F, Abell C, Huck WTS (2010) A double droplet trap system for studying mass transport across a droplet–droplet interface. Lab Chip 10:1281–1285
Barbee KD, Hsiao AP, Heller MJ, Huang XH (2009) Electric field directed assembly of high-density microbead arrays. Lab Chip 9:3268–3274
Bruzewicz DA, McGuigan AP, Whitesides GM (2008) Fabrication of a modular tissue construct in a microfluidic chip. Lab Chip 8:663–671
Di Carlo D, Aghdam N, Lee LP (2006) Single-cell enzyme concentrations, kinetics, and inhibition analysis using high-density hydrodynamic cell isolation arrays. Anal Chem 78:4925–4930
Dong YY, Xu Y, Liu ZX, Fu YF, Ohashi T, Tanaka Y, Mawatari K, Kitamori T (2011) Rapid screening swine foot-and-mouth disease virus using micro-ELISA system. Lab Chip 11:2153–2155
Duffy DC, McDonald JC, Schueller OJA, Whitesides GM (1998) Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal Chem 70:4974–4984
Hartnett JP, Kostic M (1990) Turbulent friction factor correlations for power law fluids in circular and non-circular channels. Int Commun Heat Mass 17:59–65
Iwai K, Tan WH, Ishihara H, Takeuchi S (2011) A resettable dynamic microarray device. Biomed Microdev 13:1089–1094
Kim DN, Lee Y, Koh WG (2009) Fabrication of microfluidic devices incorporating bead-based reaction and microarray-based detection system for enzymatic assay. Sensor Actuat B-Chem 137:305–312
Lee PJ, Hung PJ, Shaw R, Jan L, Lee LP (2005) Microfluidic application-specific integrated device for monitoring direct cell–cell communication via gap junctions between individual cell pairs. Appl Phys Lett 86:223902
Lien KY, Hung LY, Huang TB, Tsai YC, Lei HY, Lee GB (2011) Rapid detection of influenza A virus infection utilizing an immunomagnetic bead-based microfluidic system. Biosens Bioelectron 26:3900–3907
Lim CT, Zhang Y (2007) Bead-based microfluidic immunoassays: the next generation. Biosens Bioelectron 22:1197–1204
Matsumoto S, Yamaguchi S, Wada A, Matsui T, Ikeda M, Hamachi I (2008) Photo-responsive gel droplet as a nano- or pico-litre container comprising a supramolecular hydrogel. Chem Commun. doi:10.1039/B719004b
Morimoto Y, Tan WH, Tsuda Y, Takeuchi S (2009a) Monodisperse semi-permeable microcapsules for continuous observation of cells. Lab Chip 9:2217–2223
Morimoto Y, Tan WH, Takeuchi S (2009b) Three-dimensional axisymmetric flow-focusing device using stereolithography. Biomed Microdev 11:369–377
Nagai H, Narita Y, Ohtaki M, Saito K, Wakida SI (2007) Single-bead analysis on a disk-shaped microfluidic device using an antigen-immobilized bead. Anal Sci 23:975–979
Noda H, Kohara Y, Okano K, Kambara H (2003) Automated bead alignment apparatus using a single bead capturing technique for fabrication of a miniaturized bead-based DNA probe array. Anal Chem 75:3250–3255
Rettig JR, Folch A (2005) Large-scale single-cell trapping and imaging using microwell arrays. Anal Chem 77:5628–5634
Shi WW, Qin JH, Ye NN, Lin BC (2008) Droplet-based microfluidic system for individual Caenorhabditis elegans assay. Lab Chip 8:1432–1435
Skelley AM, Kirak O, Suh H, Jaenisch R, Voldman J (2009) Microfluidic control of cell pairing and fusion. Nat Methods 6:147–152
Sochol RD, Casavant BP, Dueck ME, Lee LP, Lin L (2011) A dynamic bead-based microarray for parallel DNA detection. J Micromech Microeng 21:054019
Song H, Tice JD, Ismagilov RF (2003) A microfluidic system for controlling reaction networks in time. Angew Chem-Int Edit 42:768–772
Studer V, Hang G, Pandolfi A, Ortiz M, Anderson WF, Quake SR (2004) Scaling properties of a low-actuation pressure microfluidic valve. J Appl Phys 95:393–398
Tan WH, Takeuchi S (2007a) A trap-and-release integrated microfluidic system for dynamic microarray applications. Proc Natl Acad Sci USA 104:1146–1151
Tan WH, Takeuchi S (2007b) Monodisperse alginate hydrogel microbeads for cell encapsulation. Adv Mater. doi:10.1002/adma.200700433
Teshima T, Ishihara H, Iwai K, Adachi A, Takeuchi S (2010) A dynamic microarray device for paired bead-based analysis. Lab Chip 10:2443–2448
Thorsen T, Maerkl SJ, Quake SR (2002) Microfluidic large-scale integration. Science 298:580–584
Torisawa Y, Chueh BH, Huh D, Ramamurthy P, Roth TM, Barald KF, Takayama S (2007) Efficient formation of uniform-sized embryoid bodies using a compartmentalized microchannel device. Lab Chip 7:770–776
Unger MA, Chou HP, Thorsen T, Scherer A, Quake SR (2000) Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 288:113–116
Wang W, Yang C, Liu YS, Li CM (2010) On-demand droplet release for droplet-based microfluidic system. Lab Chip 10:559–562
Wu LY, Di Carlo D, Lee LP (2008) Microfluidic self-assembly of tumor spheroids for anticancer drug discovery. Biomed Microdev 10:197–202
Xu J, Ahn B, Lee H, Xu L, Lee K, Panchapakesan R, Oh KW (2012) Droplet-based microfluidic device for multiple-droplet clustering. Lab Chip. doi:10.1039/c2lc20883k
Acknowledgments
We thank Yuya Morimoto at the University of Tokyo for the helpful advice on preparing monodisperse hydrogel beads. T. Tonooka and T. Teshima are supported by Research Fellowships of the Japan Society for the Promotion of Science (JSPS) for Young Scientists, Japan.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tonooka, T., Teshima, T. & Takeuchi, S. Clustering triple microbeads in a dynamic microarray for timing-controllable bead-based reactions. Microfluid Nanofluid 14, 1039–1048 (2013). https://doi.org/10.1007/s10404-012-1111-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-012-1111-7