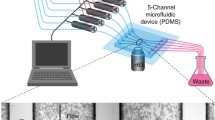
Abstract
In biology, it is not only the magnitude of a chemical stimulus that determines cellular response; it is becoming increasingly clear that the timing of the stimulus is vastly important as well. Currently there is a paucity of data regarding cell behavior under dynamic stimulation conditions that are representative of what occurs in vivo. This is, at least in part, attributed to the lack of appropriate tools for generating time-varying stimulatory signals in highly diverse patterns. Fluidics on the macro and micro scale has provided a practical platform for dynamically stimulating cells in a highly controllable manner at physiological and supra-physiological time scales (seconds to a few hours). These fluidic systems have contributed substantially to our understanding of how cells process and react to dynamic stimulatory environments; while these setups provide the means to analyze and manipulate cellular behavior in these types of environments on the single-cell level and on a high-throughput level, improvements can be made to these platforms to enhance their utility for high-impact biological investigations of temporal dynamics.







Similar content being viewed by others
References
Ando J, Ohtsuka A, Katayama Y, Araya S, Kamiya A (1990) Fluid shear stress effects on intracellular calcium concentrations in cultured vascular endothelial cells. Kokyu To Junkan 38:1107–1113
Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S (2004) Bacterial persistence as a phenotypic switch. Science 305:1622–1635
Barkai N, Leibler S (2000) Biological rhythms—circadian clocks limited by noise. Nature 403:267–268
Beebe DJ, Mensing GA, Walker GM (2002) Physics and applications of microfluidics in biology. Annu Rev Biomed Eng 4:261–286
Bennett MR, Pang WL, Ostroff NA, Baumgartner BL, Nayak S, Tsimring LS, Hasty J (2008) Metabolic gene regulation in a dynamically changing environment. Nature 454:1119–1122
Block SM, Segall JE, Berg HC (1982) Impulse responses in bacterial chemotaxis. Cell 31:215–226
Brabant G, Prank K, Schofl C (1992) Pulsatile patterns in hormone secretion. Trends Endocrinol Metab 3:183–190
Brewitt B, Clark JI (1988) Growth and transparency in the lens, an epithelial tissue, stimulated by pulses of PDGF. Science 242:777–779
Chiu DT, Orwar O (2004) Functional cell-based high-throughput drug screening. Drug Discov World 5:45–51
Dalkin AC, Haisenleder DJ, Ortolano GA, Ellis TR, Marshall JC (1989) The frequency of gonadotropin releasing hormone stimulation differentially regulates gonadotropin subunit messenger ribonucleic acid expression. Endocrinology 125:917–924
Dolmetsch RE, Xu KL, Lewis RS (1998) Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392:933–936
Duffy DC, McDonald JC, Schueller OJA, Whitesides GM (1998) Rapid prototyping of microfluidic systems in poly(dimetheylsiloxane). Anal Chem 70:4974–4984
Estes DJ, Memarsadeghi S, Lundy SK, Marti F, Mikol DD, Fox DA, Mayer M (2008) High-throughput profiling of ion channel activity in primary human lympochytes. Anal Chem 80:3728–3735
Fertig N, Blick RH, Behrends JC (2002) Whole cell patch clamp recording performed on a planar glass chip. Biophys J 6:3056–3062
Futai N, Gu W, Song JW, Takayama S (2006) Handheld recirculation system and customized media for microfluidic cell culture. Lab Chip 6:149–154
Gambacciani M, Liu JH, Swartz WH, Teuros VS, Yen SSC, Rasmussen DD (1987) Intrinsic pulsatility of luteinizing-hormone release from the human pituitary in-vitro. Neuroendocrinology 25:402–406
Goldbeter A (1996) Biochemical oscillations and cellular rhythms. The molecular bases of periodic and chaotic behaviour. Cambridge University Press, Cambridge
Goldbeter A (2008) Biological rhythms: clocks for all times. Curr Biol 18:R751–R753
Gomez-Sjoberg R, Leyrat AA, Pirone DM, Chen CS, Quake SR (2007) Versatile, fully automated, microfluidic cell culture system. Anal Chem 79:8557–8563
Griffith LG, Naughton G (2002) Tissue engineering—current challenges and expanding opportunities. Science 295:1009–1016
Groisman A, Lobo C, Cho HJ, Campbell JK, Dufour YS, Stevens AM, Levchenko A (2005) A microfluidic chemostat for experiments with bacterial and yeast cells. Nat Methods 2:685–689
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+-indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450
Gu W, Zhu XY, Futai N, Cho BS, Takayama S (2004) Computerized microfluidic cell culture using elastomeric channels and Braille displays. Proc Natl Acad Sci USA 101:15861–15866
Gunawardena J (2008) Signals and systems: towards a systems biology of signal transduction. Proc IEEE 96:1386–1397
Harrison DJ, Manz A, Fan ZH, Ludi H, Widmer HM (1992) Capillary electrophoresis and sample injection systems integrated on a planar glass chip. Anal Chem 64:1926–1932
Heo YS, Cabrera LM, Song JW, Futai N, Tung YC, Smith GD, Takayama S (2006) Characterization and resolution of evaporation-mediated osmolality shifts that constrain microfluidic cell culture in poly(dimethylsiloxane) devices. Anal Chem 79:1126–1134
Hersen P, McClean MN, Mahadevan L, Ramanathan S (2008) Signal processing by the HOG MAP kinase pathway. Proc Natl Acad Sci USA 105:7165–7170
Honda A, Adams SR, Sawyer CL, Lev-Ram V, Tsien RY, Dostmann WR (2001) Spatiotemporal dynamics of guanosine 3–5-cyclic monophosphate revealed by a genetically encoded, fluorescent indicator. Proc Natl Acad Sci USA 98:2437–2442
Huang CJ, Harootunian A, Maher MP, Quan C, Raj CD, McCormack K, Numann R, Negulescu PA, Gonzalez JE (2006) Characterization of voltage-gated sodium-channel blockers by electrical stimulation and fluorescence detection of membrane potential. Nat Biotechnol 4:439–446
Huh D, Fujioka H, Tung YC, Futai N, Paine R, Grotberg JB, Takayama S (2007) Acoustically detectable cellular-level lung injury induced by fluid mechanical stresses in microfluidic airway systems. Proc Natl Acad Sci USA 104:18886–18891
Hung PJ, Lee PJ, Sabounchi P, Lin R, Lee LP (2005) Continuous perfusion microfluidic cell culture array for high-throughput cell-based assays. Biotechnol Bioeng 89:1–8
Hynes NE, Lane HA (2005) ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 5:341–354
Ingolia NT, Weissman JS (2008) Systems biology: reverse engineering the cell. Nature 454:1059–1062
Jares-Erijman EA, Jovin TM (2003) FRET imaging. Nat Biotechnol 21:1387–1395
Kang JH, Kim YC, Park JK (2007) Analysis of pressure-driven air bubble elimination in a microfluidic device. Lab Chip 8:176–178
Khetani SR, Bhatia SN (2006) Engineering tissues for in vitro applications. Curr Opin Biotechnol 17:524–531
King KR, Wang SH, Irimia D, Jayaraman A, Toner M, Yarmush ML (2007) A high-throughput microfluidic real-time gene expression living cell array. Lab Chip 7:77–85
King KR, Wang S, Jayaraman A, Yarmush ML, Toner M (2008) Microfluidic flow-encoded switching for parallel control of dynamic cellular microenvironments. Lab Chip 8:107–116
Kitano M, Nakaya M, Nakamura T, Nagata S, Matsuda M (2008) Imaging of Rab5 activity identifies essential regulators of phagosome maturation. Nature 453:241–245
Knobil E (1981a) Patterns of hormonal signals and hormone action. N Engl J Med 305:1582–1583
Knobil E (1981b) Patterns of hypophysiotropic signals and gonadotropin-secretion in the rhesus-monkey. Biol Reprod 24:44–49
Kreke MR, Goldstein AS (2004) Hydrodynamic shear stimulates osteocalcin expression but not proliferation of bone marrow stromal cells. Tissue Eng 10:780–788
Kupzig S, Walker SA, Cullen PJ (2005) The frequencies of calcium oscillations are optimized for efficient calcium-mediated activation of Ras and the ERK/MAPK cascade. Proc Natl Acad Sci USA 102:7577–7582
Kurokawa K, Mochizuki N, Ohba Y, Mizuno H, Miyawaki A, Matsuda M (2001) A pair of fluorescent resonance energy transfer-based probes for tyrosine phosphorylation of the CrkII adaptor protein in vivo. J Biol Chem 276:31305–31310
Laurent G (2002) Olfactory network dynamics and the coding of multidimensional signals. Nat Rev Neurosci 3:884–895
Li YX, Goldbeter A (1989) Frequency specificity in intercellular communication. Biophys J 55:125–145
Li YX, Goldbeter A (1992) Pulsatile signaling in intercellular signaling in intercellular communication. Periodic stimuli are more efficient than random or chaotic signals in a model based on receptor desensitization. Biophys J 61:161–171
Lu H, Koo LY, Wang WCM, Lauffenburger DA, Griffith LG, Jensen KF (2004) Microfluidic shear devices for quantitative analysis of cell adhesion. Anal Chem 18:5257–5264
Martiel JL, Goldbeter A (1987) A model based on receptor desensitization for cyclic-AMP signaling in dictyostelium cells. Biophys J 52:807–828
Mehta G, Kiel MJ, Lee JW, Kotov N, Linderman JJ, Takayama S (2007) Polyelectrolyte–clay–protein layer films on microfluidic PDMS bioreactor surfaces for primary murine bone marrow cultures. Adv Funct Mater 17:2701–2709
Melin J, Quake SR (2007) Microfluidic large-scale integration: the evolution of design rules for biological automation. Annu Rev Biophys Biomol Struct 36:213–231
Mettetal JM, Muzzey D, Gomez-Uribe C, van Oudenaarden A (2008) The frequency dependence of osmo-adaptation in Saccharomyces cerevisiae. Science 319:482–484
Miyawaki A (2003) Visualization of the spatial and temporal dynamics of intracellular signaling. Dev Cell 4:295–305
Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY (1997) Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 388:882–887
Miyawaki A, Griesbeck O, Heim R, Tsien RY (1999) Dynamic and quantitative Ca2+ measurements using improved cameleons. Proc Natl Acad Sci USA 96:2135–2140
Mochizuki N, Yamashita S, Kurokawa K, Ohba Y, Nagai T, Miyawaki A, Matsuda M (2001) Spatio-temporal images of growth-factor-induced activation of Ras and Rap1. Nature 411:1065–1068
Morin JG, Hastings JW (1971) Energy transfer in a bioluminescent system. J Cell Physiol 77:313–318
Nagai Y, Miyazaki M, Aoki R, Zama T, Inouye S, Hirose K, Iino M, Hagiwara M (2002) A fluorescent indicator for visualizing cAMP-induced phosphorylation in vivo. Nat Biotechnol 18:313–316
Nikolaev VO, Bunemann M, Hein L, Hannawacker A, Lohse MJ (2004) Novel single chain cAMP sensors for receptor induced signal propagation. J Biol Chem 279:37215–37218
Norstedt G, Palmiter R (1984) Secretory rhythm of growth hormone regulates sexual differentiation of mouse liver. Cell 36:805–812
Novak B, Tyson JJ (1993) Numerical analysis of a comprehensive model of M-phase control in Xenopus oocyte extracts and intact embryos. J Cell Sci 106:1153–1168
Olofsson J, Pihl J, Sinclair J, Sahlin E, Karlsson K, Orwar O (2004) A microfluidics approach to the problem of creating separate solution environments accessible from macroscopic volumes. Anal Chem 76:4968–4976
Olofsson J, Bridle H, Sinclair J, Granfeldt D, Sahlin E, Orwar O (2005) A chemical waveform synthesizer. Proc Natl Acad Sci USA 102:8097–8102
Paliwal S, Wang CJ, Levchenko A (2008) Pulsing cells: how fast is too fast? HFSP J 2:251–256
Persidis A (1998) Signal transduction as a drug-discovery platform. Nat Biotechnol 16:1082–1083
Riddle RC, Taylor AF, Genetos DC, Donahue HJ (2006) MAP kinase and calcium signaling mediate fluid flow-induced human mesenchymal stem cell proliferation. Am J Physiol Cell Physiol 290:776–784
Robertson A, Drage DJ, Cohen MH (1972) Control of aggregation in Dictyostelium discoideum by an external periodic pulse of cyclic adenosine monophosphate. Science 175:333–335
Saigusa T, Tero A, Nakagaki T, Kuramoto Y (2008) Amoebae anticipate periodic events. Phys Rev Lett 100:018101
Sanford KK, Earle WR, Likely GD (1948) The growth in-vitro of single isolate tissue cells. J Natl Cancer Inst 9:229–246
Sato M, Hida N, Ozawa T, Umezawa Y (2000) Fluorescent indicators for cyclic GMP based on cyclic GMP-dependent protein. Anal Chem 72:5918–5924
Sawano A, Takayama S, Matsuda M, Miyawaki A (2002) Lateral propagation of EGF signaling after local stimulation is dependent on receptor density. Dev Cell 3:245–257
Schmidt C, Mayer M, Vogel H (2000) A chip-based biosensor for the functional analysis of single ion channels. Angew Chem Int Ed Engl 39:3137–3140
Schofl C, Sanchez-Bueno A, Brabant G, Cobbold PH, Cuthbertson KS (1991) Frequency and amplitude enhancement of calcium transients by cyclic AMP in hepatocytes. Biochem J 273:799–802
Schofl C, Brabant G, Hesch RD, von zur Muhlen A, Cobbold PH, Cuthbertson KS (1993) Temporal patterns of alpha1-receptor stimulation regulate amplitude and frequency of calcium transients. Am J Physiol 265:C1030–C1036
Schofl C, Prank K, Brabant G (1994) Mechanisms of cellular processing. Trends Endocrinol Metab 5:53–59
Shapiro L, Wagner N (1989) Transferrin is an autocrine growth factor secreted by reuber H-35 cells in serum-free culture. In Vitro Cell Dev Biol 25:650–654
Shim J, Bersano-Begey TF, Zhu X, Tkaczyk A, Linderman JJ, Takayama S (2003) Micro- and nanotechnologies for studying cellular function. Curr Top Med Chem 3:687–703
Shimomura O, Saiga Y, Johnson FH (1962a) Purification and properties of aequorin, a bio- (chemi-) luminescent protein from jelly-fish, Aequorea aequorea. Fed Proc 21:401
Shimomura O, Johnson FH, Saiga Y (1962b) Extraction, purification and properties of aequorin, a bioluminescent protein from luminous hydromedusan, Aequorea. J Cell Comp Physiol 59:223–239
Steiner RA, Bremner WJ, Clifton DK (1982) Regulation of luteinizing-hormone pulse frequency and amplitude by testosterone in the adult male rate. Endocrinology 111:2055–2061
Tay LH, Griesbeck O, Yue DT (2007) Live cell transforms between calcium transients and FRET responses for a troponin C based calcium sensor. Biophys J 93:4031–4040
Terry SC, Jerman JH, Angell JB (1979) Gas-chromatographic air analyzer fabricated on a silicon wafer. IEEE Trans Electron Devices 26:1880–1886
Ting AY, Kain KH, Klemke RL, Tsien RY (2001) Genetically encoded fluorescent reporters of protein tyrosine kinase activities in living cells. Proc Natl Acad Sci USA 98:15003–15008
Tolic IM, Moseklide E, Sturis J (2000) Modeling the insulin-glucose feedback system: the significance of pulsatile insulin secretion. J Theor Biol 207:361–375
Tourovskaia A, Figueroa-Masot X, Folch A (2005) Differentiation-on-a-chip: a microfluidic platform for long-term cell culture studies. Lab Chip 5:14–19
Tsang VL, Bhatia SN (2006) Fabrication of three-dimensional tissues. Tissue Eng 103:189–205
Van Couter E, Refetoff S (1985) Evidence for 2 subtypes of Cushing’s disease based on the analysis of episodic cortisol secretion. N Engl J Med 312:1342–1349
Verkman AS, Chao AC, Hartmann T (1992) Hormonal regulation of Cl transport in polar airway epithelia measured by a fluorescent indicator. Am J Physiol 262:C23–C31
Walker GM, Zeringue HC, Beebe DJ (2004) Microenvironment design consideration for cellular scale studies. Lab Chip 4:91–97
Weigle DS, Koerker DJ, Goodner CJ (1984) Pulsatile glucagon delivery enhances glucose production by perifused rat hepatocytes. Am J Physiol 247:E564–E568
Whitesides GM, Ostuni E, Takayama S, Jiang XY, Ingber DE (2001) Soft lithography in biology and biochemistry. Annu Rev Biomed Eng 3:335–373
Wildt L, Hausler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E (1981) Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin-secretion in the rhesus monkey. Endocrinology 109:376–385
Zhang J, Ma Y, Taylor SS, Tsien RY (2001) Genetically encoded reporters of protein kinase A activity reveal impact of substrate tethering. Proc Natl Acad Sci USA 98:14997–15002
Zhang J, Campbell RE, Ting AY, Tsien RY (2002) Creating new fluorescent probes for cell biology. Nat Rev Mol Cell Biol 3:906–918
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jovic, A., Howell, B. & Takayama, S. Timing is everything: using fluidics to understand the role of temporal dynamics in cellular systems. Microfluid Nanofluid 6, 717–729 (2009). https://doi.org/10.1007/s10404-009-0413-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-009-0413-x