Abstract
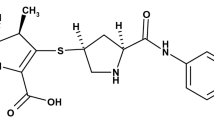
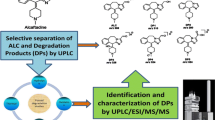
A novel stability-indicating HPLC method for the quantitative determination of impurities in meropenem raw material was established and fully validated for the further improvement of the official monograph in Pharmacopeia. The method was carried out on a Shim-Pack CLC-ODS column with UV detection at 220 nm. The influences of different band columns, composition ratio of mobile phase, gradient elution procedure, and pH value of mobile phase on separation of impurities in meropenem were investigated. The parameters specificity, precision, linearity, range, accuracy, limit of detection, limit of quantitation, and robustness were studied according to the regulatory guidelines recommended by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). The linear regression analysis results of EP impurity A and EP impurity B show that there is a good linear relationship between response and concentration, and the correlation coefficients are 0.9999 and 1.0000, respectively. The accuracy of EP impurity A and EP impurity B is between 90 and 104%. Through analysis, the LOD and LOQ values of EP impurities A and B were measured and found to be 0.22 and 0.74 µg mL-1, respectively. Moreover, three unknown degradation impurities in meropenem were identified by two-dimensional liquid chromatography tandem with high-resolution ion trap/time-of-flight mass spectrometry in electrospray ionization mode. Compared with the current method in Pharmacopeia, the developed method had greatly improved the separation and detection ability of impurities. The established method was highly specific, sensitive, and accurate for reliable routine quality control of meropenem raw material. The structures of unknown impurities were characterized based on the MS/MS data.







Similar content being viewed by others
References
Pfaller MA, Jones RN (1997) A review of the in vitro activity of meropenem and comparative antimicrobial agents tested against 30,254 aerobic and anaerobic pathogens isolated world wide. Diagn Microbiol Infect Dis. https://doi.org/10.1016/S0732-8893(97)00065-5
Blumer JL (1997) Meropenem: evaluation of a new generation carbapenem. Int J Antimicrob Agents. https://doi.org/10.1016/S0924-8579(96)00347-0
Wiseman LR, Wagstaff AJ, Brogden RN, Bryson HM (1995) Meropenem A review of its antibacterial activity, pharmacokinetic properties and clinical efficacy. Drugs. https://doi.org/10.2165/00003495-199550010-00007
Sun C, Wu J, Pan Y (2009) Characterization of novel hydrolysis products of carbapenems by electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom. https://doi.org/10.1002/rcm.4240
Barnes J, Caldwell M, Curel P et al (1995) Meropenem: a new carbapenem with potential for treating bacterial meningitis. Drugs and Therapy Perspect. https://doi.org/10.2165/00042310-199506100-00001
Bradley JS (1997) Meropenem: a new, extremely broad spectrum beta-lactam antibiotic for serious infections in pediatrics. Pediatr Infect Dis J. https://doi.org/10.1097/00006454-199703000-00002
Pharmacopoeia of the People’s Republic of China (2020) Edition 2020, Chemical Industry Press
United States Pharmacopoeia (2019) 43th edn. United States Pharmacopeial Convention
European Pharmacopoeia (2019) 10th edn. Council of Europe Strasbourg
Japanese Pharmacopoeia (2016) 17th edn. Ministry of Health, Labour and Welfare
ICH Harmonised Tripartite Guideline (2006) Impurities in new drug substances Q3A (R2). In: International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human use.
Ren XJ, Zhou JJ, Hu FF, Wang J (2020) Study of the impurity profile and characteristic fragmentation of Δ3-isomers in cephapirin sodium using dual liquid chromatography coupled with ion trap/time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. https://doi.org/10.1002/rcm.8948
Liu GJ, Zhu BQ, Ren XJ, Wang J (2020) Characterization of 28 unknown impurities in 16-membered macrolides by liquid chromatography coupled with ion trap/time-of-flight mass spectrometry. J Pharm Biomed Anal. https://doi.org/10.1016/j.jpba.2020.113324
Rao KT, Rao LV, Kandepi V (2020) A novel validated eco-friendly RP-UHPLC method for assay and related substances in meropenem. Indian J Chem 59:1148
Elragehy NA, Abdel-Moety EM, Hassan NY, Rezk MR (2008) Stability-indicating determination of meropenem in presence of its degradation products. Talanta. https://doi.org/10.1016/j.talanta.2008.06.045
Barbosa FS, Pezzi LC, Tsao M, Mendez A et al (2020) Stability in clinical use and stress testing of meropenem antibiotic by direct infusion ESI-Q-TOF: quantitative method and identification of degradation products. J Pharm Biomed Anal. https://doi.org/10.1016/j.jpba.2019.112973
Mendez A, Chagastelles P, Palma E, Nardi N, Schapoval E (2008) Thermal and alkaline stability of meropenem: degradation products and cytotoxicity. Int J Pharm. https://doi.org/10.1016/j.ijpharm.2007.08.023
Takeuchi Y, Sunagawa M, Isobe Y, Hamazume Y, Noguchi T (1995) Stability of a 1β-methylcarbapenem antibiotic, meropenem (SM-7338) in aqueous solution. Chem Pharm Bull. https://doi.org/10.1248/cpb.43.689
Cai SY, Hu CQ (2005) Chromatographic determination of polymerized impurities in meropenem. J Pharm Biomed Anal. https://doi.org/10.1016/j.jpba.2004.11.023
ICH Harmonized Tripartite Guideline (1994) Validation of Analytical Procedures: Text and Methodology Q2 (R1). In: International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human use.
ICH Harmonized Tripartite Guideline (2003) Stability Testing of New Drug Substances and Products Q1A (R2). In: International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human use.
Acknowledgements
This study was supported by the Science and Technology project of Zhejiang Food and Drug Administration in 2020 (No. 202001), and National Science and Technology Major Projects for “Major New Drugs Innovation and Development” (No. 2017ZX09101001, Beijing, China).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ren, X., Ye, J., Chen, X. et al. Development of a Novel HPLC Method for the Analysis of Impurities in Meropenem and Identification of Unknown Impurities by 2D LC-IT-TOF MS. Chromatographia 84, 937–947 (2021). https://doi.org/10.1007/s10337-021-04081-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-021-04081-4