Abstract
Objectives
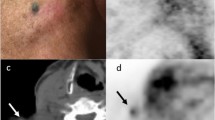
Electron paramagnetic resonance (EPR) oximetry using particulate materials allows repeatable measurements of oxygen in tissues. However, the materials identified so far are not medical devices, thus precluding their immediate use in clinical studies. The aim of this study was to assess the magnetic properties of Carbo-Rep®, a charcoal suspension used as a liquid marker for preoperative tumor localization.
Materials and methods
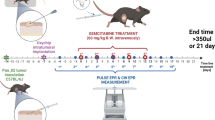
Calibration curves (EPR linewidth as a function of pO2) were built using 9-GHz EPR spectrometry. The feasibility of performing oxygen measurements was examined in vivo by using a low-frequency (1 GHz) EPR spectrometer and by inducing ischemia in the gastrocnemius muscle of mice or by submitting rats bearing tumors to different oxygen-breathing challenges.
Results
Paramagnetic centers presenting a high oxygen sensitivity were identified in Carbo-Rep®. At 1 GHz, the EPR linewidth varied from 98 to 426 µT in L-band in nitrogen and air, respectively. The sensor allowed repeated measurements of oxygen over 6 months in muscles of mice. Subtle variations of tumor oxygenation were monitored in rats when switching gas breathing from air to carbogen.
Discussion
The magnetic properties of Carbo-Rep® are promising for its future use as oxygen sensor in clinical EPR oximetry.







Similar content being viewed by others
References
Springett R, Swartz HM (2007) Measurements of oxygen in vivo: overview and perspectives on methods to measure oxygen within cells and tissues. Antioxid Redox Signal 9:1295–1301
Vikram DS, Zweier JL, Kuppusamy P (2007) Methods for noninvasive imaging of tissue hypoxia. Antioxid Redox Signal 9:1745–1756
Colliez F, Gallez B, Jordan BF (2017) Assessing tumor oxygenation for predicting outcome in radiation oncology: a review of studies correlating tumor hypoxic status and outcome in the preclinical and clinical settings. Front Oncol 7:10
Bussink J, Kaanders JH, van der Kogel AJ (2003) Tumor hypoxia at the micro-regional level: clinical relevance and predictive value of exogenous and endogenous hypoxic cell markers. Radiother Oncol 67:3–15
Peeters SG, Zegers CM, Yaromina A, Van Elmpt W, Dubois L, Lambin P (2015) Current preclinical and clinical applications of hypoxia PET imaging using 2-nitroimidazoles. Q J Nucl Med Mol Imaging 59:39–57
Griffiths JR, Robinson SP (1999) The OxyLite: a fibre-optic oxygen sensor. Br J Radiol 72:627–630
Vaupel P, Höckel M, Mayer A (2007) Detection and characterization of tumor hypoxia using pO2 histography. Antioxid Redox Signal 9:1221–1235
Price JM, Robinson SP, Koh DM (2013) Imaging hypoxia in tumours with advanced MRI. Q J Nucl Med Mol Imaging 57:257–270
Howe FA, Robinson SP, McIntyre DJ, Stubbs M, Griffiths JR (2001) Issues in flow and oxygenation dependent contrast (FLOOD) imaging of tumours. NMR Biomed 14:497–506
Baudelet C, Gallez B (2002) How does blood oxygen level-dependent (BOLD) contrast correlate with oxygen partial pressure (pO2) inside tumors? Magn Reson Med 48:980–986
Baudelet C, Gallez B (2004) Current issues in the utility of blood oxygen level dependent MRI for the assessment of modulations in tumor oxygenation. Curr Med Imaging Rev 1:229–243
O’Connor JP, Boult JK, Jamin Y, Babur M, Finegan KG, Williams KJ, Little RA, Jackson A, Parker GJ, Reynolds AR, Waterton JC, Robinson SP (2016) Oxygen-enhanced MRI accurately identifies, quantifies, and maps tumor hypoxia in preclinical cancer models. Cancer Res 76:787–795
Cao-Pham TT, Tran LB, Colliez F, Joudiou N, El Bachiri S, Grégoire V, Levêque P, Gallez B, Jordan BF (2016) Monitoring tumor response to carbogen breathing by oxygen-sensitive magnetic resonance parameters to predict the outcome of radiation therapy: a preclinical study. Int J Radiat Oncol Biol Phys 96:149–160
Kodibagkar VD, Wang X, Mason RP (2008) Physical principles of quantitative nuclear magnetic resonance oximetry. Front Biosci 13:1371–1384
Jordan BF, Cron GO, Gallez B (2009) Rapid monitoring of oxygenation by 19F magnetic resonance imaging: simultaneous comparison with fluorescence quenching. Magn Reson Med 61:634–638
Swartz HM, Clarkson RB (1998) The measurement of oxygen in vivo using EPR techniques. Phys Med Biol 43:1957–1975
Gallez B, Baudelet C, Jordan BF (2004) Assessment of tumor oxygenation by electron paramagnetic resonance: principles and applications. NMR Biomed 17:240–262
Krishna MC, Matsumoto S, Yasui H, Saito K, Devasahayam N, Subramanian S, Mitchell JB (2012) Electron paramagnetic resonance imaging of tumor pO2. Radiat Res 177:376–386
Ahmad R, Kuppusamy P (2010) Theory, instrumentation, and applications of electron paramagnetic resonance oximetry. Chem Rev 110:3212–3236
Epel B, Halpern HJ (2015) In vivo pO2 imaging of tumors: oxymetry with very low-frequency electron paramagnetic resonance. Methods Enzymol 564:501–527
Liu KJ, Gast P, Moussavi M, Norby SW, Vahidi N, Walczak T, Wu M, Swartz HM (1993) Lithium phthalocyanine: a probe for electron paramagnetic resonance oximetry in viable biological systems. Proc Natl Acad Sci USA 90:5438–5442
Pandian RP, Parinandi NL, Ilangovan G, Zweier JL, Kuppusamy P (2003) Novel particulate spin probe for targeted determination of oxygen in cells and tissues. Free Radic Biol Med 35:1138–1148
Jordan BF, Baudelet C, Gallez B (1998) Carbon-centered radicals as oxygen sensors for in vivo electron paramagnetic resonance: screening for an optimal probe among commercially available charcoals. MAGMA 7:121–129
Lan M, Beghein N, Charlier N, Gallez B (2004) Carbon blacks as EPR sensors for localized measurements of tissue oxygenation. Magn Reson Med 51:1272–1278
Khan N, Mupparaju S, Hou H, Williams BB, Swartz HM (2012) Repeated assessment of orthotopic glioma pO2 by multi-site EPR oximetry: a technique with the potential to guide therapeutic optimization by repeated measurements of oxygen. J Neurosci Methods 204:111–117
Ilangovan G, Liebgott T, Kutala VK, Petryakov S, Zweier JL, Kuppusamy P (2004) EPR oximetry in the beating heart: myocardial oxygen consumption rate as an index of postischemic recovery. Magn Reson Med 51:835–842
Jordan BF, Kimpalou JZ, Beghein N, Dessy C, Feron O, Gallez B (2004) Contribution of oxygenation to BOLD contrast in exercising muscle. Magn Reson Med 52:391–396
James PE, Miyake M, Swartz HM (1999) Simultaneous measurement of NO and pO2 from tissue by in vivo EPR. Nitric Oxide 3:292–301
He G, Shankar RA, Chzhan M, Samouilov A, Kuppusamy P, Zweier JL (1999) Noninvasive measurement of anatomic structure and intraluminal oxygenation in the gastrointestinal tract of living mice with spatial and spectral EPR imaging. Proc Natl Acad Sci USA 96:4586–4591
James PE, Bacic G, Grinberg OY, Goda F, Dunn JF, Jackson SK, Swartz HM (1996) Endotoxin-induced changes in intrarenal pO2, measured by in vivo electron paramagnetic resonance oximetry and magnetic resonance imaging. Free Radic Biol Med 21:25–34
Krzic M, Sentjurc M, Kristl J (2001) Improved skin oxygenation after benzyl nicotinate application in different carriers as measured by EPR oximetry in vivo. J Control Release 70:203–211
Van Eyck AS, Jordan BF, Gallez B, Heilier JF, Van Langendonckt A, Donnez J (2009) Electron paramagnetic resonance as a tool to evaluate human ovarian tissue reoxygenation after xenografting. Fertil Steril 92:374–381
Vériter S, Gianello P, Igarashi Y, Beaurin G, Ghyselinck A, Aouassar N, Jordan B, Gallez B, Dufrane D (2014) Improvement of subcutaneous bioartificial pancreas vascularization and function by coencapsulation of pig islets and mesenchymal stem cells in primates. Cell Transplant 23:1349–1364
Desmet CM, Lafosse A, Vériter S, Porporato PE, Sonveaux P, Dufrane D, Levêque P, Gallez B (2015) Application of electron paramagnetic resonance (EPR) oximetry to monitor oxygen in wounds in diabetic models. PLoS One 10:e0144914
Gallez B, Jordan BF, Baudelet C, Misson PD (1999) Pharmacological modifications of the partial pressure of oxygen in murine tumors: evaluation using in vivo EPR oximetry. Magn Reson Med 42:627–630
Jordan BF, Grégoire V, Demeure RJ, Sonveaux P, Feron O, O’Hara J, Vanhulle VP, Delzenne N, Gallez B (2002) Insulin increases the sensitivity of tumors to irradiation: involvement of an increase in tumor oxygenation mediated by a nitric oxide-dependent decrease of the tumor cells oxygen consumption. Cancer Res 62:3555–3561
Crokart N, Radermacher K, Jordan BF, Baudelet C, Cron GO, Grégoire V, Beghein N, Bouzin C, Feron O, Gallez B (2005) Tumor radiosensitization by antiinflammatory drugs: evidence for a new mechanism involving the oxygen effect. Cancer Res 65:7911–7916
Ansiaux R, Baudelet C, Jordan BF, Beghein N, Sonveaux P, De Wever J, Martinive P, Grégoire V, Feron O, Gallez B (2005) Thalidomide radiosensitizes tumors through early changes in the tumor microenvironment. Clin Cancer Res 11:743–750
Hou H, Khan N, Grinberg OY, Yu H, Grinberg SA, Lu S, Demidenko E, Steffen RP, Swartz HM (2007) The effects of Efaproxyn (efaproxiral) on subcutaneous RIF-1 tumor oxygenation and enhancement of radiotherapy-mediated inhibition of tumor growth in mice. Radiat Res 168:218–225
Crokart N, Jordan BF, Baudelet C, Cron GO, Hotton J, Radermacher K, Grégoire V, Beghein N, Martinive P, Bouzin C, Feron O, Gallez B (2007) Glucocorticoids modulate tumor radiation response through a decrease in tumor oxygen consumption. Clin Cancer Res 13:630–635
Diepart C, Karroum O, Magat J, Feron O, Verrax J, Calderon PB, Grégoire V, Leveque P, Stockis J, Dauguet N, Jordan BF, Gallez B (2012) Arsenic trioxide treatment decreases the oxygen consumption rate of tumor cells and radiosensitizes solid tumors. Cancer Res 72:482–490
O’Hara JA, Goda F, Liu KJ, Bacic G, Hoopes PJ, Swartz HM (1995) The pO2 in a murine tumor after irradiation: an in vivo electron paramagnetic resonance oximetry study. Radiat Res 144:222–229
Crokart N, Jordan BF, Baudelet C, Ansiaux R, Sonveaux P, Grégoire V, Beghein N, DeWever J, Bouzin C, Feron O, Gallez B (2005) Early reoxygenation in tumors after irradiation: determining factors and consequences for radiotherapy regimens using daily multiple fractions. Int J Radiat Oncol Biol Phys 63:901–910
Cron GO, Beghein N, Crokart N, Chavée E, Bernard S, Vynckier S, Scalliet P, Gallez B (2005) Changes in the tumor microenvironment during low-dose-rate permanent seed implantation iodine-125 brachytherapy. Int J Radiat Oncol Biol Phys 63:1245–1251
Fujii H, Sakata K, Katsumata Y, Sato R, Kinouchi M, Someya M, Masunaga S, Hareyama M, Swartz HM, Hirata H (2008) Tissue oxygenation in a murine SCC VII tumor after X-ray irradiation as determined by EPR spectroscopy. Radiother Oncol 86:354–360
Salikhov I, Walczak T, Lesniewski P, Khan N, Iwasaki A, Comi R, Buckey J, Swartz HM (2005) EPR spectrometer for clinical applications. Magn Reson Med 54:1317–1320
Swartz HM, Williams BB, Zaki BI, Hartford AC, Jarvis LA, Chen EY, Comi RJ, Ernstoff MS, Hou H, Khan N, Swarts SG, Flood AB, Kuppusamy P (2014) Clinical EPR: unique opportunities and some challenges. Acad Radiol 21:197–206
Swartz HM, Williams BB, Hou H, Khan N, Jarvis LA, Chen EY, Schaner PE, Ali A, Gallez B, Kuppusamy P, Flood AB (2016) Direct and repeated clinical measurements of pO2 for enhancing cancer therapy and other applications. Adv Exp Med Biol 923:95–104
Gallez B, Mäder K (2000) Accurate and sensitive measurements of pO2 in vivo using low frequency EPR spectroscopy: how to confer biocompatibility to the oxygen sensors. Free Radic Biol Med 29:1078–1084
Swartz HM, Liu KJ, Goda F, Walczak T (1994) India ink: a potential clinically applicable EPR oximetry probe. Magn Reson Med 31:229–232
Charlier N, Beghein N, Gallez B (2004) Development and evaluation of biocompatible inks for the local measurement of oxygen using in vivo EPR. NMR Biomed 17:303–310
Gallez B, Debuyst R, Liu KJ, Demeure R, Dejehet F, Swartz HM (1996) Development of biocompatible implants of fusinite for in vivo EPR oximetry. MAGMA 4:71–75
He J, Beghein N, Ceroke P, Clarkson RB, Swartz HM, Gallez B (2001) Development of biocompatible oxygen-permeable films holding paramagnetic carbon particles: evaluation of their performance and stability in EPR oximetry. Magn Reson Med 46:610–614
Dinguizli M, Jeumont S, Beghein N, He J, Walczak T, Lesniewski PN, Hou H, Grinberg OY, Sucheta A, Swartz HM, Gallez B (2006) Development and evaluation of biocompatible films of polytetrafluoroethylene polymers holding lithium phthalocyanine crystals for their use in EPR oximetry. Biosens Bioelectron 21:1015–1022
Dinguizli M, Beghein N, Gallez B (2008) Retrievable micro-inserts containing oxygen sensors for monitoring tissue oxygenation using EPR oximetry. Physiol Meas 29:1247–1254
Eteshola E, Pandian RP, Lee SC, Kuppusamy P (2009) Polymer coating of paramagnetic particulates for in vivo oxygen-sensing applications. Biomed Microdevices 11:379–387
Pandian RP, Meenakshisundaram G, Bratasz A, Eteshola E, Lee SC, Kuppusamy P (2010) An implantable teflon chip holding lithium naphthalocyanine microcrystals for secure, safe, and repeated measurements of pO2 in tissues. Biomed Microdevices 12:381–387
Hou H, Khan N, Gohain S, Kuppusamy ML, Kuppusamy P (2018) Pre-clinical evaluation of OxyChip for long-term EPR oximetry. Biomed Microdevices 20:29
Baudelet C, Gallez B (2004) Effect of anesthesia on the signal intensity in tumors using BOLD-MRI: comparison with flow measurements by Laser Doppler flowmetry and oxygen measurements by luminescence-based probes. Magn Reson Imaging 22:905–912
Walsh JC, Lebedev A, Aten E, Madsen K, Marciano L, Kolb HC (2001) The clinical importance of assessing tumor hypoxia: relationship of tumor hypoxia to prognosis and therapeutic opportunities. Antioxid Redox Signal 21:1516–1554
Vaupel P, Kelleher DK, Höckel M (2001) Oxygen status of malignant tumors: pathogenesis of hypoxia and significance for tumor therapy. Semin Oncol 28:29–35
Horsman MR, Wouters BG, Joiner MC, Overgaard J (2009) The oxygen effect and fractionated radiotherapy. In: Joiner M, van der Kogel A (eds) Basic clinical radiobiology, 4th edn. H. Arnold, London, pp 207–216
Wouters BG, Korintzinsky M (2009) The tumor micro-environment and cellular hypoxia responses. In: Joiner M, van der Kogel A (eds) Basic clinical radiobiology, 4th edn. H. Arnold, London, pp 217–232
Khan N, Williams BB, Hou H, Li H, Swartz HM (2007) Repetitive tissue pO2 measurements by electron paramagnetic resonance oximetry: current status and future potential for experimental and clinical studies. Antioxid Redox Signal 9:1169–1182
Swartz HM, Hou H, Khan N, Jarvis LA, Chen EY, Williams BB, Kuppusamy P (2014) Advances in probes and methods for clinical EPR oximetry. Adv Exp Med Biol 812:73–79
Flood AB, Wood VA, Swartz HM (2017) Using India ink as a sensor for oximetry: evidence of its safety as a medical device. Adv Exp Med Biol 977:297–312
Vahidi N, Clarkson RB, Liu KJ, Norby SW, Wu M, Swartz HM (1994) In vivo and in vitro EPR oximetry with fusinite: a new coal-derived, particulate EPR probe. Magn Reson Med 31:139–146
James PE, Grinberg OY, Goda F, Panz T, O’Hara JA, Swartz HM (1997) Gloxy: an oxygen-sensitive coal for accurate measurement of low oxygen tensions in biological systems. Magn Reson Med 38:48–58
Swartz HM, Khan N, Buckey J, Comi R, Gould L, Grinberg O, Hartford A, Hopf H, Hou H, Hug E, Iwasaki A, Lesniewski P, Salikhov I, Walczak T (2004) Clinical applications of EPR: overview and perspectives. NMR Biomed 17:335–351
Desmet CM, Préat V, Gallez B (2018) Nanomedicines and gene therapy for the delivery of growth factors to improve perfusion and oxygenation in wound healing. Adv Drug Deliv Rev 129:262–284
Matsuo M, Matsumoto S, Mitchell JB, Krishna MC, Camphausen K (2014) Magnetic resonance imaging of the tumor microenvironment in radiotherapy: perfusion, hypoxia, and metabolism. Semin Radiat Oncol 24:210–217
Epel B, Maggio M, Pelizzari C, Halpern HJ (2017) Electron paramagnetic resonance pO2 image tumor oxygen-guided radiation therapy optimization. Adv Exp Med Biol 977:287–296
Acknowledgements
The authors wish to thank Dr. Fabienne Danhier for performing the particle-size measurements.
Funding
The work was supported by the National Cancer Institute (NCI, USA) PO1CA190193, the Actions de Recherches Concertées ARC 14/19-058, and the Fonds Joseph Maisin. This research used the EPR facilities of the technological platform Nuclear and Electron Spin Technologies of the Louvain Drug Research Institute.
Author information
Authors and Affiliations
Contributions
CMD, LBAT, PD: data acquisition, analysis, and interpretation; manuscript drafting; critical revision. BG: study conception and design; data analysis and interpretation; manuscript drafting; critical revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human rights
This article does not contain any studies with human participants performed by any of the authors
Statement on the welfare of animals
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (authorization 2014/UCL/MD/026).
Rights and permissions
About this article
Cite this article
Desmet, C.M., Tran, L.B.A., Danhier, P. et al. Characterization of a clinically used charcoal suspension for in vivo EPR oximetry. Magn Reson Mater Phy 32, 205–212 (2019). https://doi.org/10.1007/s10334-018-0704-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-018-0704-x