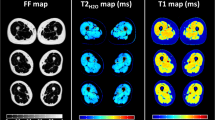
Abstract
Objectives
To evaluate the combination of a fat–water separation method with an automated segmentation algorithm to quantify the intermuscular fatty-infiltrated fraction, the relaxation times, and the microscopic fatty infiltration in the normal-appearing muscle.
Materials and methods
MR acquisitions were performed at 1.5T in seven patients with facio-scapulo-humeral dystrophy and eight controls. Disease severity was assessed using commonly used scales for the upper and lower limbs. The fat–water separation method provided proton density fat fraction (PDFF) and relaxation times maps (T 2* and T 1). The segmentation algorithm distinguished adipose tissue and normal-appearing muscle from the T 2* map and combined active contours, a clustering analysis, and a morphological closing process to calculate the index of fatty infiltration (IFI) in the muscle compartment defined as the relative amount of pixels with the ratio between the number of pixels within IMAT and the total number of pixels (IMAT + normal appearing muscle).
Results
In patients, relaxation times were longer and a larger fatty infiltration has been quantified in the normal-appearing muscle. T 2* and PDFF distributions were broader. The relaxation times were correlated to the Vignos scale whereas the microscopic fatty infiltration was linked to the Medwin-Gardner-Walton scale. The IFI was linked to a composite clinical severity scale gathering the whole set of scales.
Conclusion
The MRI indices quantified within the normal-appearing muscle could be considered as potential biomarkers of dystrophies and quantitatively illustrate tissue alterations such as inflammation and fatty infiltration.




Similar content being viewed by others
References
Mercuri E, Pichiecchio A, Allsop J, Messina S, Pane M, Muntoni F (2007) Muscle MRI in inherited neuromuscular disorders: past, present, and future. J Magn Reson Imaging 25(2):433–440
Wren TA, Bluml S, Tseng-Ong L, Gilsanz V (2008) Three-point technique of fat quantification of muscle tissue as a marker of disease progression in Duchenne muscular dystrophy: preliminary study. AJR Am J Roentgenol 190(1):W8–W12
Hu HH, Bornert P, Hernando D, Kellman P, Ma J, Reeder S, Sirlin C (2012) ISMRM workshop on fat-water separation: insights, applications and progress in MRI. Magn Reson Med 68(2):378–388
Mercuri E, Jungbluth H, Muntoni F (2005) Muscle imaging in clinical practice: diagnostic value of muscle magnetic resonance imaging in inherited neuromuscular disorders. Curr Opin Neurol 18(5):526–537
Akima H, Lott D, Senesac C, Deol J, Germain S, Arpan I, Bendixen R, Lee Sweeney H, Walter G, Vandenborne K (2012) Relationships of thigh muscle contractile and non-contractile tissue with function, strength, and age in boys with Duchenne muscular dystrophy. Neuromuscul Disord 22(1):16–25
Cea G, Bendahan D, Manners D, Hilton-Jones D, Lodi R, Styles P, Taylor DJ (2002) Reduced oxidative phosphorylation and proton efflux suggest reduced capillary blood supply in skeletal muscle of patients with dermatomyositis and polymyositis: a quantitative 31P-magnetic resonance spectroscopy and MRI study. Brain 125(Pt 7):1635–1645
Fischer D, Kley RA, Strach K, Meyer C, Sommer T, Eger K et al (2008) Distinct muscle imaging patterns in myofibrillar myopathies. Neurology 71(10):758–765
Kornblum C, Lutterbey G, Bogdanow M, Kesper K, Schild H, Schroder R, Wattjes MP (2006) Distinct neuromuscular phenotypes in myotonic dystrophy types 1 and 2: a whole body highfield MRI study. J Neurol 253(6):753–761
Mattei JP, Fur YL, Cuge N, Guis S, Cozzone PJ, Bendahan D (2006) Segmentation of fascias, fat and muscle from magnetic resonance images in humans: the DISPIMAG software. Magn Reson Mater Phy 19(5):275–279
Positano V, Christiansen T, Santarelli MF, Ringgaard S, Landini L, Gastaldelli A (2009) Accurate segmentation of subcutaneous and intermuscular adipose tissue from MR images of the thigh. J Magn Reson Imaging 29(3):677–684
Dixon WT (1984) Simple proton spectroscopic imaging. Radiology 153(1):189–194
Bydder M, Yokoo T, Hamilton G, Middleton MS, Chavez AD, Schwimmer JB, Lavine JE, Sirlin CB (2008) Relaxation effects in the quantification of fat using gradient echo imaging. Magn Reson Imaging 26(3):347–359
Guiu B, Petit JM, Loffroy R, Ben Salem D, Aho S, Masson D, Robin I, Vergès B, Hillon P, Cercueil JP, Krausé D (2009) Quantification of liver fat content: comparison of triple-echo chemical shift gradient-echo imaging and in vivo proton MR spectroscopy. Radiology 250(1):95–102
Hussain HK, Chenevert TL, Londy FJ, Gulani V, Swanson SD, McKenna BJ, Appelman HD, Adusumilli S, Greenson JK, Conjeevaram HS (2005) Hepatic fat fraction: MR imaging for quantitative measurement and display-early experience. Radiology 237(3):1048–1055
Leporq B, Ratiney H, Cavassila S, Pilleul F, Beuf O (2010). Fat content quantification errors using multiple gradient echo imaging: A phantom and simulation study. In: ISMRM-ESMRMB Joint Annual Meeting, Stockholm, p 2581
Hines CD, Frydrychowicz A, Hamilton G, Tudorascu DL, Vigen KK, Yu H, McKenzie CA, Sirlin CB, Brittain JH, Reeder SB (2011) T1 independent, T2* corrected chemical shift based fat-water separation with multi-peak fat spectral modeling is an accurate and precise measure of hepatic steatosis. J Magn Reson Imaging 33(4):873–881
Leporq B, Lambert SA, Ronot M, Vilgrain V, Van Beers BE (2014) Quantification of the triglyceride fatty acid composition with 3.0 T MRI. NMR Biomed 27(10):1211–1221
Leporq B, Ratiney H, Pilleul F, Beuf O (2013) Liver fat volume fraction quantification with fat and water T1 and T2* estimation and accounting for NMR multiple components in patients with chronic liver disease at 1.5 and 3.0 T. Eur Radiol 23(8):2175–2186
Meisamy S, Hines CD, Hamilton G, Sirlin CB, McKenzie CA, Yu H, Brittain JH, Reeder SB (2011) Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology 258(3):767–775
Yu H, Shimakawa A, Hines CD, McKenzie CA, Hamilton G, Sirlin CB, Brittain JH, Reeder SB (2011) Combination of complex-based and magnitude-based multiecho water-fat separation for accurate quantification of fat-fraction. Magn Reson Med 66(1):199–206
Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB (2008) Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med 60(5):1122–1134
Yokoo T, Bydder M, Hamilton G, Middleton MS, Gamst AC, Wolfson T, Hassanein T, Patton HM, Lavine JE, Schwimmer JB, Sirlin CB (2009) Nonalcoholic fatty liver disease: diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5 T. Radiology 251(1):67–76
Florence JM, Pandya S, King WM, Robison JD, Signore LC, Wentzell M, Province MA (1984) Clinical trials in Duchenne dystrophy. standardization and reliability of evaluation procedures. Phys Ther 64(1):41–45
Brooke MH, Griggs RC, Mendell JR, Fenichel GM, Shumate JB, Pellegrino RJ (1981) Clinical trial in Duchenne dystrophy. I. The design of the protocol. Muscle Nerve 4(3):186–197
Strothotte S et al (2010) Enzyme replacement therapy with alglucosidase alfa in 44 patients with late-onset glycogen storage disease type 2: 12-month results of an observational clinical trial. J Neurol 257(1):91–97
Gold GE, Han E, Stainsby J, Wright GA, Brittain J, Beaulieu C (2004) Musculoskeletal MRI at 3.0T: relaxation times and image contrast. Am J Neuroradiol 183:343–350
de Bazelaire CM, Duhamel GD, Rofsky NM, Alsop DC (2004) MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology 230(3):652–659
Lareau-Trudel E, Le Troter A, Ghattas B, Pouget J, Attarian S, Bendahan D, Salort-Campana E (2015) Muscle quantitative MR imaging and clustering analysis in patients with facioscapulohumeral muscular dystrophy type 1. PLoS One 10(7):e0132717
Willis TA, Hollingsworth KG, Coombs A, Sveen ML, Andersen S, Stojkovic T (2013) Quantitative muscle MRI as an assessment tool for monitoring disease progression in LGMD2I: a multicentre longitudinal study. PLoS One 8(8):e70993
Janiczek RL, Gambarota G, Sinclair CD, Yousry TA, Thornton JS, Golay X, Newbould RD (2011) Simultaneous T2 and lipid quantitation using IDEAL-CPMG. Magn Reson Med 66(5):1293–1302
Morrow JM, Sinclair CD, Fischmann A, Reilly MM, Hanna MG, Yousry TA, Thornton JS (2014) Reproducibility, and age, body-weight and gender dependency of candidate skeletal muscle MRI outcome measures in healthy volunteers. Eur Radiol 24(7):1610–1620
Alizai H, Nardo L, Karampinos DC, Joseph GB, Yap SP, Baum T, Krug R, Majumdar S, Link TM (2012) Comparison of clinical semi-quantitative assessment of muscle fat infiltration with quantitative assessment using chemical shift-based water/fat separation in MR studies of the calf of post-menopausal women. Eur Radiol 22(7):1592–1600
Machann J, Häring H, Schick F, Stumvoll M (2004) Intramyocellular lipids and insulin resistance. Diabetes Obes Metab 6(4):239–248
Hollingsworth KG, Garrood P, Eagle M, Bushby K, Straub V (2013) Magnetic resonance imaging in Duchenne muscular dystrophy: longitudinal assessment of natural history over 18 months. Muscle Nerve 48(4):586–588
Kan HE, Scheenen TW, Wohlgemuth M, Klomp DW, van Loosbroek-Wagenmans I, Padberg GW, Heerschap A (2009) Quantitative MR imaging of individual muscle involvement in facioscapulohumeral muscular dystrophy. Neuromuscul Disord 19(5):357–362
Arpan I, Forbes SC, Lott DJ, Senesac CR, Daniels MJ, Triplett WT et al (2013) T2 mapping provides multiple approaches for the characterization of muscle involvement in neuromuscular diseases: a cross-sectional study of lower leg muscles in 5-15-year-old boys with Duchenne muscular dystrophy. NMR Biomed 26(3):320–328
Cassidy FH, Yokoo T, Aganovic L, Hanna RF, Bydder M, Middleton MS, Hamilton G, Chavez AD, Schwimmer JB, Sirlin CB (2009) Fatty liver disease: MR imaging techniques for the detection and quantification of liver steatosis. Radiographics 29(1):231–260
Pichiecchio A, Poloni GU, Ravaglia S, Ponzio M, Germani G, Maranzana D, Costa A, Repetto A, Tavazzi E, Danesino C, Moglia A, Bastianello S (2009) Enzyme replacement therapy in adult-onset glycogenosis II: is quantitative muscle MRI helpful? Muscle Nerve 40(1):122–125
Wokke BH, Bos C, Reijnierse M, van Rijswijk CS, Eggers H, Webb A, Verschuuren JJ, Kan HE (2013) Comparison of dixon and T1-weighted MR methods to assess the degree of fat infiltration in duchenne muscular dystrophy patients. J Magn Reson Imaging 38(3):619–624
Reeder SB, Bice EK, Yu H, Hernando D, Pineda AR (2012) On the performance of T2* correction methods for quantification of hepatic fat content. Magn Reson Med 67(2):389–404
Acknowledgements
This work was performed within the framework of the LABEX PRIMES (ANR-11-LABX-0063) of Université de Lyon, within the program “Investissements d’Avenir” (ANR-11-IDEX-0007) operated by the French National Research Agency (ANR).
Authors’ contribution
Leporq: Protocol/project development; Data analysis. Le Troter: Protocol/project development; Data analysis. Le Fur: Protocol/project development. Salort-Campana: Data collection or management. Guye: Data collection or management. Attarian: Data collection or management. Beuf: Protocol/project development. Bendahan: Data collection or management; Data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Leporq, B., Le Troter, A., Le Fur, Y. et al. Combined quantification of fatty infiltration, T 1-relaxation times and T 2*-relaxation times in normal-appearing skeletal muscle of controls and dystrophic patients. Magn Reson Mater Phy 30, 407–415 (2017). https://doi.org/10.1007/s10334-017-0616-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-017-0616-1