Abstract
Object
Cytoplasmic lipid droplets (LDs) are dynamic cellular organelles; their accumulation is associated with several cellular processes, such as cell proliferation, apoptosis and necrosis. 1H Nuclear Magnetic Resonance (NMR) spectroscopy detects resonances from lipids present in cytoplasmic (LDs); an understanding of the relationship between LD characteristics and NMR lipid signals is important.
Materials and methods
In this study, five nervous system cancer cell lines were investigated. Nile red staining was used to measure the diameter of LDs. High-resolution magic angle spinning NMR (HR-MAS) was performed on harvested cell pellets to quantify the patterns of lipid signals.
Results
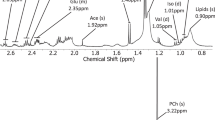
LDs were present in all five cell lines with different morphology. An average LD diameter of approximately 0.2 μm was found in all cell types. Diameter of the largest LDs varied across the cell lines. The intensity of NMR lipid signals varied greatly between cell types, and a good correlation was found between total volume of LDs and the proton NMR lipid signal intensity at 0.9 and 1.3 ppm.
Conclusion
The correlation implied that little NMR signal is detected from LDs of diameters less than approximately 0.34 μm, most likely due to restriction of rotational motion of the lipids.




Similar content being viewed by others
References
Glunde K, Artemov D, Penet M-F, Jacobs MA, Bhujwalla ZM (2009) Magnetic resonance spectroscopy in metabolic and molecular imaging and diagnosis of cancer. Chem Rev 110:3043–3059
Callot V, Galanaud D, Le Fur Y, Confort-Gouny S, Ranjeva J-P, Cozzone PJ (2008) 1H MR spectroscopy of human brain tumours: a practical approach. Eur J Radiol 67:268–274
Barba I, Cabanas ME, Arus C (1999) The relationship between nuclear magnetic resonance-visible lipids, lipid droplets, and cell proliferation in cultured C6 cells. Cancer Res 59:1861–1868
Opstad KS, Bell BA, Griffiths JR, Howe FA (2008) An investigation of human brain tumour lipids by high-resolution magic angle spinning 1H MRS and histological analysis. NMR Biomed 21:677–685
Negendank WG, Sauter R, Brown TR, Evelhoch JL, Falini A, Gotsis ED, Heerschap A, Kamada K, Lee BCP, Mengeot MM, Moser E, Padavic-Shaller KA, Sanders JA, Spraggins TA, Stillman AE, Terwey B, Vogl TJ, Wicklow K, Zimmerman RA (1996) Proton magnetic resonance spectroscopy in patients with glial tumors: a multicenter study. J Neurosurg 84:449–458
Hakumaki JM, Kauppinen RA (2000) 1H NMR visible lipids in the life and death of cells. Trends Biochem Sci 25:357–362
Remy C, Fouilhe N, Barba I, Sam-Lai E, Lahrech H, Cucurella M-G, Izquierdo M, Moreno A, Ziegler A, Massarelli R, Decorps M, Arus C (1997) Evidence that mobile lipids detected in rat brain glioma by 1H nuclear magnetic resonance correspond to lipid droplets. Cancer Res 57:407–414
Callies R, Sri-Pathmanathan RM, Ferguson DYP, Brindle KM (1993) The appearance of neutral lipid signals in the 1H NMR spectra of a myeloma cell line correlates with the induced formation of cytoplasmic lipid droplets. Magn Reson Med 29:546–550
Kuesel AC, Sutherland GR, Halliday W, Smith ICP (1994) 1H MRS of high grade astrocytomas: mobile lipid accumulation in necrotic tissue. NMR Biomed 7:149–155
Di Vito M, Lenti L, Knijn A, Iorio E, D’Agostino F, Molinari A, Calcabrini A, Stringaro A, Meschini S, Arancia G, Bozzi A, Strom R, Podo F (2001) 1H NMR-visible mobile lipid domains correlate with cytoplasmic lipid bodies in apoptotic T-lymphoblastoid cells. Biochim Biophys Acta 1530:47–66
Bickel PE, Tansey JT, Welte MA (2009) PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta Mol Cell Biol Lipids 1791:419–440
Martin S, Parton RG (2006) Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol 7:373–378
Weller RO, Clark RA, Oswald WB (1968) Stages in the formation and metabolism in intracellular lipid droplets in atherosclerosis. An electron microscopical and biochemical study. J Atheroscler Res 8:249–263
Greenberg AS, Coleman RA, Kraemer FB, McManaman JL, Obin MS, Puri V, Yan Q-W, Miyoshi H, Mashek DG The role of lipid droplets in metabolic disease in rodents and humans. J Clin Invest 121: 2102–2110
Bozza PT, Magalhães KG, Weller PF (2009) Leukocyte lipid bodies—biogenesis and functions in inflammation. Biochim Biophys Acta Mol Cell Biol Lipids 1791:540–551
Miyanari Y, Atsuzawa K, Usuda N, Watashi K, Hishiki T, Zayas M, Bartenschlager R, Wakita T, Hijikata M, Shimotohno K (2007) The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol 9:1089–1097
Nandor GT, Balazs S, Szabolcs B, Timea B, Gyorgy S, Tamas J, Andras S, Hans B, Gabor NT (2003) Lipid droplet and milk lipid globule membrane associated placental protein 17b (PP17b) is involved in apoptotic and differentiation processes of human epithelial cervical carcinoma cells. Eur J Biochem 270:1176–1188
Accioly MT, Pacheco P, Maya-Monteiro CM, Carrossini N, Robbs BK, Oliveira SS, Kaufmann C, Morgado-Diaz JA, Bozza PT, JPB Viola (2008) Lipid bodies are reservoirs of cyclooxygenase-2 and sites of prostaglandin-E2 synthesis in colon cancer cells. Cancer Res 68:1732–1740
Quintero M, Cabanas ME, Arus C (2007) A possible cellular explanation for the NMR-visible mobile lipid (ML) changes in cultured C6 glioma cells with growth. Biochim Biophys Acta 1771:31–44
Wilson M, Reynolds G, Kauppinen RA, Arvanitis TN, Peet AC (2010) A constrained least-squares approach to the automated quantitation of in vivo (1)H magnetic resonance spectroscopy data. Magn Reson Med 65:1–12
Bayet-Robert M, Loiseau D, Rio P, Demidem A, Barthomeuf C, Stepien G, Morvan D (2010) Quantitative two-dimensional HRMAS 1H-NMR spectroscopy-based metabolite profiling of human cancer cell lines and response to chemotherapy. Magn Reson Med 63:1172–1183
Murphy DJ (2001) The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog Lipid Res 40:325–438
Perez Y, Lahrech H, Cabanas ME, Barnadas R, Sabes M, Remy C, Arus C (2002) Measurement by nuclear magnetic resonance diffusion of the dimensions of the mobile lipid compartment in C6 cells. Cancer Res 62:5672–5677
Valverde-Saubí D, Candiota A, Molins M, Feliz M, Godino Ó, Dávila M, Acebes J, Arús C (2010) Short-term temperature effect on the HRMAS spectra of human brain tumor biopsies and their pattern recognition analysis. Magn Reson Mater Phy 23:203–215
Kauppinen RA, Nissinen T, Karkkainen AM, Pirttila TR, Palvimo J, Kokko H, Williams SR (1992) Detection of thymosin beta 4 in situ in a guinea pig cerebral cortex preparation using 1H NMR spectroscopy. J Biol Chem 267:9905–9910
Acknowledgments
1H NMR experiments were carried out in the Henry Wellcome Building for Biomolecular NMR Spectroscopy at the University of Birmingham and we are grateful for the support of the staff at this facility. The work was partly funded by the Medical Research Council, UK (Grant G0601327), the Andrew McCartney Trust Fund for Brain Tumor Research, Birmingham Children’s Hospital Research Foundation and the Brain and Nervous System Tumor Research Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pan, X., Wilson, M., McConville, C. et al. The size of cytoplasmic lipid droplets varies between tumour cell lines of the nervous system: a 1H NMR spectroscopy study. Magn Reson Mater Phy 25, 479–485 (2012). https://doi.org/10.1007/s10334-012-0315-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-012-0315-x