Abstract
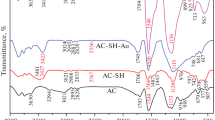
This study is to develop a carbon-based adsorbent containing multiple functional ligands for effective removal of lead ions from aqueous media. Activated carbon was oxidized by nitric acid, followed by chlorination with thionyl chloride and reaction with ethylenediamine. Modified activated carbon (MAC) was characterized using scanning electron microscopy in conjunction of energy dispersive spectroscopy (SEM-EDS), Fourier transform infrared spectroscopy (FT-IR), and potentiometric titration. Surface characterizations confirmed that carboxyl, amine, and chlorine functional groups were effectively introduced onto the carbon surface by the treatments. The modifications lowered the pH at the point of zero charge (pHpzc) from 9.6 to 2.55 and resulted in more negatively charged surfaces. Adsorptive experiments showed that aqueous Pb removal by MAC was faster, with a 62% higher capacity than the original activated carbon (60.2 vs. 37.2 mg g−1).






Similar content being viewed by others
References
Aggarwal D, Goyal M, Bansal RC (1999) Adsorption of chromium by activated carbon from aqueous solution. Carbon 37:1989–1997. doi:10.1016/S0008-6223(99)00072-X
Badmus MAO, Audu TOK, Anyata BU (2007) Removal of lead ion from industrial wastewaters by activated carbon prepared from Periwinkle shells (Typanotonus fuscatus). Turk J Eng Environ Sci 31:251–263
Gu ZM, Deng BL (2007) Arsenic sorption and redox transformation on iron-impregnated ordered mesoporous carbon. Appl Organomet Chem 21:750–757. doi:10.1002/aoc.1283
Li J, Bai RB (2002) Mechanisms of lead adsorption on chitosan/PVA hydrogel beads. Langmuir 18:9765–9770. doi:10.1021/la025917l
Li N, Bai RB (2006) Highly enhanced adsorption of lead ions on chitosan granules functionalized with poly (acrylic acid). Ind Eng Chem Res 45:7897–7904. doi:10.1021/ie060514s
Mallikarjuna N, Venkataraman A (2003) Adsorption of Pb2+ ions on nanosized g-Fe2O3: formation of surface ternary complexes on ligand complexation. Talanta 60:139–147. doi:10.1016/S0039-9140(03)00119-X
Newland LW, Daun KA (1982) Part B, Anthropogenic compounds. In: Hutzinger O (ed) Handbook of environmental chemistry, vol 3. Springer, New York, p 1
Petrovic M, Kastelan-Macan M, Horvat AJM (1999) Interactive sorption of metal ions and humic acids onto mineral particles. Water Air Soil Pollut 111:41–56. doi:10.1023/A:1005084802830
Przepiorski J (2006) Enhanced adsorption of phenol from water by ammonia-treated activated carbon. J Hazard Mater B135:453–456. doi:10.1016/j.jhazmat.2005.12.004
Silva AR, Castro B, Figueiredo JL (2002) Immobilisation of amine-functionalised nickel (II) schiff base complexes onto activated carbon treated with thionyl chloride. Microporous Mesoporous Mater 55:275–284. doi:10.1016/S1387-1811(02)00429-8
Socrates G (1994) Infrared characteristic group frequencies. Wiley, Chichester
Swiatkowski A, Pakula M, Biniak S, Walczyk M (2004) Influence of the surface chemistry of modified activated carbon on its electrochemical behaviour in the presence of lead (II) ions. Carbon 42:3057–3069. doi:10.1016/j.carbon.2004.06.043
World Health Organization (2006) Guidelines for drinking-water quality, 3rd edn. World Health Organization, Geneva, p 54
Wu SH, Pendleton P (2001) Adsorption of anionic surfactant by activated carbon: effect of surface chemistry, ionic strength, and hydrophobicity. J Colloid Interface Sci 243:306–315
Yantasee W, Lin YH, Fryxell GE (2004) Selective removal of copper (II) from aqueous solutions using fine-grained activated carbon functionalized with amine. Ind Eng Chem Res 43:2759–2764
Acknowledgments
Authors would thank Dr. Keith Goyne at University of Missouri for his technical support in FT-IR analyses. Financial supports for this research were provided by the USDA-CSREES (MOX-YANG), the USEPA-NCER-STAR (RD831071) to Lincoln University of Missouri, and the US-DOE (DE-FC26-02NT41607) to University of Missouri.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, J., Yang, J. & Deng, B. Ethylenediamine-modified activated carbon for aqueous lead adsorption. Environ Chem Lett 8, 277–282 (2010). https://doi.org/10.1007/s10311-009-0217-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-009-0217-y