Abstract
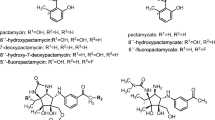
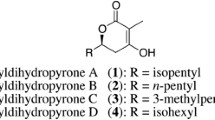
Phoslactomycins (PLMs) represent an unusual structural class of natural products secreted by various streptomycetes, containing an α,β-unsaturated δ-lactone, an amino group, phosphate ester, conjugated diene and a cyclohexane ring. Phosphazomycins, phospholines and leustroducsins contain the same structural moieties, varying only in the acyl substituent at the C-18 hydroxyl position. These compounds possess either antifungal or antitumor activities or both. The antitumor activity of the PLM class of compounds has been attributed to a potent and selective inhibition of protein phosphatase 2A (PP2A). The cysteine-269 residue of PP2Ac-subunit has been shown to be the site of covalent modification by PLMs. In this article, we review previous work on the isolation, structure elucidation and biological activities of PLMs and related compounds and current status of our work on both PLM stability and genetic manipulation of the biosynthetic process. Our work has shown that PLM B is surprisingly stable in solution, with a pH optimum of 6. Preliminary biosynthetic studies utilizing isotopically labeled shikimic acid and cyclohexanecarboxylic acid (CHC) suggested PLM B to be a polyketide-type antibiotic synthesized using CHC as a starter unit. Using a gene (chcA) from a set of CHC-CoA biosynthesis genes from Streptomyces collinus as a probe, a 75 kb region of 29 ORFs encoding PLM biosynthesis was located in the genome of Streptomyces sp. strain HK803. Analysis and subsequent manipulation of plmS 2 and plmR 2 in the gene cluster has allowed for rational engineering of a strain that produces only one PLM analog, PLM B, at ninefold higher titers than the wild type strain. A strain producing PLM G (the penultimate intermediate in PLMs biosynthesis) has also been generated. Current work is aimed at selective in vitro acylation of PLM G with various carboxylic acids and a precursor-directed biosynthesis in a chcA deletion mutant with the aim of generating novel PLM analogs.








Similar content being viewed by others
References
Boger DL, Ichikawa S, Zhong W (2001) Total synthesis of fostriecin (CI-920). J Am Chem Soc 123:4161–4167
Bradford D (1996) Molecular mechanisms of the protein serine/threonine phosphatases. Trends Biochem Sci 21:407–412
Buck SB, Hardouin C, Ichikawa S, Soenen DR, Gauss C-M, Hwang I, Swingle MR, Bonness KM, Honkanen RE, Boger DL (2003) Fundamental role of the fostriecin unsaturated lactone and implications for selective protein phosphatase inhibition. J Am Chem Soc 125:15694–15695
Cropp TA, Wilson DJ, Reynolds KA (2000) Identification of a cyclohexylcarbonyl CoA biosynthetic gene cluster and application in the production of doramectin. Nat Biotechnol 18:980–983
Das Choudhuri S, Ayers S, Soine WH, Reynolds KA (2005) A pH-stability study of phoslactomycin B and analysis of the acid and base degradation products. J Antibiot 58:573–582
Esumi T, Okamoto N, Hatakeyama S (2002) Versatile enantiocontrolled synthesis of (+)-fostriecin. Chem Commun (Camb) 24:3042–3043
Fujii K, Maki K, Kanai M, Shibasaki M (2003) Formal catalytic asymmetric total synthesis of fostriecin. Org Lett 5:733–736
Fushimi S, Nishikawa S, Shimazu A, Seto H (1989) Studies on new phosphate ester antifungal antibiotics phoslactomycins. I. Taxonomy, fermentation, purification and biological activities. J Antibiot 42:1019–1025
Fushimi S, Furihata K, Seto H (1989) Studies on new phosphate ester antifungal antibiotics phoslactomycins. II. Structure elucidation of phoslactomycins A to F. J Antibiot 42:1026–1036
Ghatge MS, Reynolds KA (2005) The plmS2-encoded Cytochrome P450 monooxygenase mediates hydroxylation of phoslactomycin B in Streptomyces sp. strain HK803. J Bacteriol 187:7970–7976
Gust B, Challis GL, Fowler K, Kieser T, Chater KF (2003) PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc Natl Acad Sci USA 100:1541–1546
de Jong RS, Mulder NH, Uges DR, Sleijfer DT, Hoppener FJ, Groen HJ, Willemse PH, van der Graaf WT, de Vries EG (1999) Phase I and pharmacokinetic study of the topoisomerase II catalytic inhibitor fostriecin. Br J Cancer 79:882–887
Katz L (1997) Manipulation of modular polyketide synthases. Chem Rev 97:2557–2575
Kawada M, Kawatsu M, Masuda T, Ohba S, Amemiya M, Kohama T, Ishizuka M, Takeuchi T (2003) Specific inhibitors of protein phosphatase 2A inhibit tumor metastasis through augmentation of natural killer cells. Int Immunopharmacol 3:179–188
Kim BS, Cropp TA, Beck BJ, Sherman DH, Reynolds KA (2002) Biochemical evidence for an editing role of thioesterase II in the biosynthesis of the polyketide pikromycin. J Biol Chem 277:48028–48034
Kohama T, Enokita R, Okazaki T, Miyaoka H, Torikata A, Inukai M, Kaneko I, Kagasaki T, Sakaida Y, Satoh A, Shiraishi A (1993) Novel microbial metabolites of the phoslactomycins family induce production of colony-stimulating factors by bone marrow stromal cells. I. Taxonomy, fermentation and biological properties. J Antibiot 46:1503–1511
Kohama T, Nakamura T, Kinoshita T, Kaneko I, Shiraishi A (1993) Novel microbial metabolites of the phoslactomycins family induce production of colony-stimulating factors by bone marrow stromal cells. II. Isolation, physico-chemical properties and structure determination. J Antibiot 46:1512–1519
Kohama T, Katayama T, Inukai M, Maeda H, Shiraishi A (1994) Augmentation of host resistance against bacterial infection by treatment with leustroducsin B, a new CSF inducer. Microbiol Immunol 38:741–745
Kohama T, Maeda H, Sakai JI, Shiraishi A, Yamashita K (1996) Leustroducsin B, a new cytokine inducer derived from an actinomycetes, induces thrombocytosis in mice. J Antibiot (Tokyo) 49:91–94
Leopold WR,Shillis JL, Mertus AE, Nelson JM, Roberts BJ, Jackson RC (1984) Anticancer activity of the structurally novel antibiotic CI-920 and its analogues. Cancer Res 44:1928–1932
Leopold WR, Mertus AE, Nelson JM, Roberts BJ (1983) In vivo activity of the novel anticancer antibiotic PD 110,161. Proc Am Assoc Cancer Res 24:320
Lewy DS, Gauss CM, Soenen DR, Boger DL (2002) Fostriecin: chemistry and biology. Curr Med Chem 9:2005–2032
Lowden PA, Bohm GA, Staunton J, Leadlay PF (1996) The nature of the starter unit for the rapamycin polyketide synthase. Angew Chem Int Ed Eng 35:2249–2251
Lowden PA, Wilkinson B, Bohm GA, Handa S, Floss HG, Leadlay PF, Staunton J (2001) Origin and true nature of the starter unit for the rapamycin polyketide synthase. Angew Chem Int Ed Engl 40:777–779
Lu H, Tsai SC, Khosla C, Cane DE (2002) Expression, site-directed mutagenesis, and steady state kinetic analysis of the terminal thioesterase domain of the methymycin/picromycin polyketide synthase. Biochemistry 41:12590–12597
Miyashita K, Ikejiri M, Kawasaki H, Maemura S, Imanishi T (2002) Total synthesis of fostriecin (CI-920) via a convergent route. Chem Commun 7:742–743
Moore BS, Cho H, Casati R, Kennedy E, Reynolds KA, Mocek U, Beale JM, Floss HG (1993) Biosynthetic studies on ansatrienin A. Formation of the cyclohexanecarboxylic acid moiety. J Am Chem Soc 115:5254–5266
Moore BS, Poralla K, Floss HG (1993) Biosynthesis of the cyclohexanecarboxylic acid starter unit of ω-cyclohexyl fatty acids in Alicyclobacillus acidocaldarius. J Am Chem Soc 115:5267–5274
Ozasa T, Suzuki K, Sasamata M, Tanaka K, Kobori M, Kadota S, Nagai K, Saito T, Watanabe S, Iwanami M (1989) Novel antitumor antibiotic phospholine. 1. Production, isolation and characterization. J Antibiot 42:1331–1338
Ozasa T, Tanaka K, Sasamata M, Kaniwa H, Shimizu M, Matsumoto H, Iwanami M (1989) Novel antitumor antibiotic phospholine. 2. Structure determination. J Antibiot 42:1339–1343
Palaniappan N, Kim BS, Sekiyama Y, Osada H, Reynolds KA (2003) Enhancement and selective production of phoslactomycin B, a protein phosphatase IIa inhibitor, through identification and engineering of the corresponding biosynthetic gene cluster. J Biol Chem 278:35552–35557
Patton SM, Ashton T. Cropp, Reynolds KA (2000) A novel Δ3,Δ2 enoyl CoA isomerase involved in the biosynthesis of the cyclohexanecarboxylic acid-derived moiety of the polyketide ansatrienin A. Biochemistry 39:7595–7604
Reddy YK, Falck JR (2002) Asymmetric total synthesis of (+)-fostriecin. Org Lett 4:969–971
Reynolds KA, Wallace KK, Handa S, Brown MS, McArthur HAI, Floss HG (1997) Biosynthesis of the shikimate-derived starter unit of the immunosuppressant ascomycin: Stereochemistry of the 1,4-conjugate elimination. J Antibiot 50:701–703
Sekiyama Y, Palaniappan N, Reynolds KA, Osada H (2003) Biosynthesis of phoslactomycins: cyclohexanecarboxylic acid as the starter unit. Tetrahedron 59:7465–7471
Shen B (2004) Accessing natural products by combinatorial biosynthesis. Sci STKE 225:pe14
Shibata T, Kurihara S, Oikawa T, Ohkawa N, Shimazaki N, Sasagawa K, Kobayashi T, Kohama T, Asai F, Shiraishi A, Sugimura Y (1995) Preparation of leustroducsin H and the structure-activity relationship of its derivatives. J Antibiot (Tokyo) 48:1518–1520
Shibata T, Kurihara S, Yoda K, Haruyama H (1995) Absolute configuration of leustroducsins. Tetrahedron 51:11999–12012
Shimada K, Kaburagi Y, Fukuyama T (2003) Total synthesis of leustroducsin B. J Am Chem Soc 125:4048–4049
Shimada K, Koishi R, Kohama T (2004) Leustroducsins, low molecular weight thrombopoietic agents: discovery, biological properties and total synthesis. Annu Rep Sankyo Res Lab 56:11–28
Stampwala SS, Bunge RH, Hurley TR, Willmer NE, Brankiewicz AJ, Steinman CE, Smitka TA, French JC (1983) Novel antitumor agents CI-920, PD 113,270 and PD 113,271. II. Isolation and characterization. J Antibiot 36:1601–1605
Teruya T, Simizua S, Kanoha N, Osada H (2005) Phoslactomycin targets cysteine-269 of the protein phosphatase 2A catalytic subunit in cells. FEBS Lett 579:2463–2468
Tomiya T, Uramoto M, Isono K (1990) Isolation and structure of phosphazomycin C. J Antibiot 43:118–121
Tsai SC, Lu H, Cane DE, Khosla C, Stroud RM (2002) Insights into channel architecture and substrate specificity from crystal structures of two macrocycle-forming thioesterases of modular polyketide synthases. Biochemistry 41:12598–12606
Tunac JB, Graham BD, Dobson WE (1983) Novel antitumor agents CI-920, PD 113,270 and PD 113,271. I. Taxonomy, fermentation and biological properties. J Antibiot 36:1595–1600
Uramoto M, Shen YC, Takizawa N, Kusakabe H, Isono K (1985) A new antifungal antibiotic, phosphazomycin A. J Antibiot 38:665–668
Usui T, Marriott G, Inagaki M, Swarup G, Osada H (1999) Protein phosphatase 2A inhibitors, phoslactomycins. Effects on the cytoskeleton in NIH/3T3 cells. J Biochem 125:960–965
Wallace KK, Reynolds KA, Koch K, McArthur HAI, Brown MS, Wax RG, Moore BS (1994) Biosynthetic studies of ascomycin (FK520): formation of the (1R,3R,4R)-3,4-dihydroxycyclohexanecarboxylic acid-derived moiety. J Am Chem Soc 116:11600–11601
Wang P, Denoya CD, Morgenstern MR, Skinner DD, Wallace KK, DiGate R, Patton S, Banavali N, Schuler G, Speedie MK, Reynolds KA (1996) Cloning and characterization of the gene encoding 1-cyclohexenylcarbonyl CoA reductase from Streptomyces collinus. J Bacteriol 178:6873–6881
Wang YG, Kobayashi Y (2002) Formal total synthesis of fostriecin. Org Lett 4:4615–4618
Xue Y, Wilson D, Zhao L, Liu H, Sherman DH (1998) Hydroxylation of macrolactones YC-17 and narbomycin is mediated by the pikC-encoded cytochrome P450 in Streptomyces venezuelae. Chem Biol 5:661–667
Acknowledgments
Our work on PLM stability and biosynthesis is supported by grant AI51629 from the National Institutes of Health. Streptomyces sp. strain HK803 was kindly provided by Hiroyuki Osada (Riken, Japan).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghatge, M., Palaniappan, N., Das Choudhuri, S. et al. Genetic manipulation of the biosynthetic process leading to phoslactomycins, potent protein phosphatase 2A inhibitors. J IND MICROBIOL BIOTECHNOL 33, 589–599 (2006). https://doi.org/10.1007/s10295-006-0116-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-006-0116-1