Abstract
Species of scorpionfly (Mecoptera) in the family Panorpidae perform wing-waving behaviors, whereby they rotate their front and rear wings at the same time. Previous studies have suggested that a male, which carries food for use as nuptial gifts for females, performs the wing-waving behavior when the male gives the gift to a female or competes with other males. However, when and how the wing-waving behavior occurs during a series of nuptial giftings and male–male competitions have not been investigated. Therefore, we here observed the role of wing-waving behavior during the processes of giving nuptial gifts and male–male competition in the Japanese scorpionfly Panorpa japonica in the laboratory and field. Unlike previous studies, only males performed wing-waving behavior toward females, while females did not exhibit the behavior in the wild. Also, males always performed wing-waving behavior before male–male competition. After a male–male competition, winner males continued wing-waving behavior, but loser males never performed the behavior against the winner male. A comparison of wing-waving behaviors before competitions between winner and loser males showed that the frequencies of wing-waving behaviors were higher in winner than in loser males. The present results suggest that the wing-waving behavior functions in the inter-sexual and intra-sexual selection in P. japonica. Digital video images related to the article are available at http://www.momo-p.com/showdetail-e.php?movieid=momo210513pj01a and http://www.momo-p.com/showdetail-e.php?movieid=momo210513pj02a and http://www.momo-p.com/showdetail-e.php?movieid=momo210513pj03a.
Similar content being viewed by others
Introduction
Many species use parts of the body, such as wings or legs, for visual display during courtship dances (e.g., Andersson 1994; Miller and Svensson 2014). For example, the bird of paradise (Paradisaeidae) is famous for colorful feather displays during courtship dances (Scholes 2008). In the peacock spider (Maratus sp.), courtship dances using the legs and abdominal flap (and they wave their midlegs during courtship) have significant impact on the mating success of males (Madeline et al. 2015). Visual displays such as vibrating wings during courtship have been reported in many other insect species: Drosophila melanogaster (Cowling and Burnet 1981), Rhagoletis pomonella (Prokopy and Bush 1973), Megaloprepus caerulatus (Schultz and Fincke 2009), and Campoplex capitator (Benelli et al. 2019).
Nuptial gift giving is a mating behavior in which males provide gifts, such as food and nutritious secretions, to females before or during mating (Thornhill and Alcock 1983). Males of scorpionfly (Panorpidae) species attract females by a sex pheromone and provide nuptial gifts, such as a dead insect or nutritious saliva secretions, to females during courtship and mating (e.g., Sauer et al. 1997, Sato and Fujiyama 2018; Thornhill and Sauer 1992). A female allows the male to mate only when she is eating the gift (Sauer et al. 1997, Thornhill and Alcock 1983). Also, scorpionfly males fight each other for nuptial gifts.
Scorpionflies are known to perform a wing-waving (= flashing) behavior in which they move their wings up and down (Byers and Thornhill 1983). Magnier and Montgomery (2017) conducted a field experiment with the North American scorpionfly Panorpa debilis and discovered that the males and females use wing-waving behavior to protect a food resource against other insect species who are competing for it. Therefore, they suggested that the wing-waving behavior of P. debilis works as a visual display to other species. However, they did not observe whether the wing-waving behavior was used in courtship or at what time, probably because P. debilis only mates at night (Thornhill 1981).
Although another previous study using North American scorpionflies confirmed that males showed wing-waving behavior towards other males and females during the nuptial gift process (Thornhill 1981), there are no detailed studies on the relationships between wing-waving behavior and nuptial gifts in courtship, and between wing-waving behavior and male–male competition.
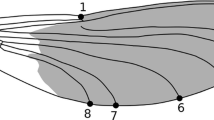
The Japanese scorpionfly, Panorpa japonica, has two black bands in the forewings and hind wings (Tsutsumi 2010), and this species also shows wing-waving behavior (Fig. 1, RI, personal observation). In the P. japonica, males assume a pheromone-releasing posture near bait and give it to an approaching female as food for a nuptial gift before mating (Thornhill 1992a). Males of P. japonica often perform male–male competitions, in which males use the ends of their abdomen graspers, over food for nuptial gifts and for females (Thornhill 1992b). In P. japonic’s male–male competition involves butting a contestant with the head, grappling with the large genital claspers, and pinching a body part of a competitor with the sharp points of the genital claspers (Thornhill 1992a). The winner of the male–male competition remains in the food, and the loser waits near the winner after withdrawing from the contested food (Thornhill 1992a). P. japonica males use three alternative mating strategies: nuptial gifts, forced mating, and feeding mating (Thornhill 1992a, Ishihara and Miyatake 2020). Males mainly use a dead insect as a nuptial gift (Thornhill 1992a). Males of P. japonica secures food for nuptial gifts for several hours (Thornhill 1992b, RI, personal observation). At the end of mating with a nuptial gift, males check their food and release pheromone again to wait for females to visit (RI, personal observation). Mating of P. japonica can be observed in the daytime (Kurokawa et al. 2012) unlike P. debilis (Magnier and Montgomery 2017). Therefore, P. japonica is an ideal material to research the relationship between wing-waving behavior and courtship behaviors or male–male competitions.
A previous study reported that satellite males of P. japonica who had lost a male–male competition waited around the winners of the competitions and attempted to force mating with females attracted by the winner males (Thornhill 1992a). In laboratory experiments with P. japonica, it was reported that males with larger fluctuating asymmetry (FA) in the forewings adopted a feeding mating approach rather than a nuptial gift (Ishihara and Miyatake 2020).
Thus, we hypothesized that the wing-waving behavior of P. japonica is used for inter- and intra-sexual displays during nuptial gift giving. In the present study, we thus examined the relationships between wing-waving behavior and courtship behaviors when giving nuptial gifts, and between wing-waving behavior and male–male competition, in the laboratory and field.
Materials and methods
P. japonica, like other Panorpidae, feeds mainly on dead insects, and males that come to feed release pheromones after they eat a little (Thornhill 1992b). Females attracted to the male pheromone copulate when they begin to feed on the male’s food (Thornhill 1992b).
Behavioral patterns observed in the present study were categorized into three categories as follows: (1) “nuptial gift”; a female approaches a male keeping food for nuptial gift and develops into nuptial gift (Fig. 2 S1 Electronic supplementary material: http://www.momo-p.com/showdetail-e.php?movieid=momo210513pj01a). (2) “male–male competition”; a male approaches a male carrying food for nuptial gift and develops into male–male competition (Fig. 3 S2 Electronic supplementary material: http://www.momo-p.com/showdetail-e.php?movieid=momo210513pj02a). (3) “satellite”; a male defeated by male–male competition takes a satellite tactic, and then a satellite male attempts to reinvade the prey, as described below, and the prey males intercept the satellite males (Fig. 4 S3 Electronic supplementary material: http://www.momo-p.com/showdetail-e.php?movieid=momo210513pj03a).
A male attempting nuptial gifting on a female visiting for food (http://www.momo-p.com/showdetail-e.php?movieid=momo210513pj01a)
Males that keep food for nuptial gift intercepting males that come for food (male–male competition) (http://www.momo-p.com/showdetail-e.php?movieid=momo210513pj02a)
A defeated male in a male–male competition, trying to reenter the bait, and a male intercepting it (satellite). The loser male is running away from the winner male’s attack (http://www.momo-p.com/showdetail-e.php?movieid=momo210513pj03a)
Laboratory experiments
Adults (40 males and 40 females) of P. japonica were collected from Handayama Mountain, Okayama City, Japan (latitude: 34°6 ‘N, longitude: 133°9’E) from May 1 to 15, 2017. The experiment was conducted for one week after the collection. Each adult was reared in a plastic container (10 cm diameter × 8 cm height) in a chamber maintained at 25 ± 2 °C and 16L:8D until the experiment. A piece of mealworm (Tenebrio molitor; weight: 0.2 g; length: 5 mm) was given once every two days.
Male and female (a pair) were placed in a plastic cylinder (10 cm diameter × 10 cm height) in which a piece of mealworm (0.2 g) had been placed on the bottom cotton whose surface is lightly moistened. After the pair was settled, mating behaviors were recorded with a video camera (HDR-PJ590V, Sony, Tokyo, Japan). The mating duration was defined as the period from the moment the male grabbed the female’s abdomen to the moment the male released the female. Each male and female pair was used for only a single observation. We observed 40 pairs in this experiment. In the present study, when mating was confirmed, we recorded the presence or absence of wing-waving behavior, and number and duration of wing-waving behavior acts.
Field experiments
The wing-waving behavior experiments were conducted from 9 am to 5 pm at the following two points: (1) from April 20 to May 19, 2019, and from April 18 to May 6, 2020, at a bank of the Asahikawa River, Okayama City(34 º6 ‘8 “ ‘N, 133º9 ‘3” ‘E), and 2) from May 8 to 26, 2020 at a road on Tsuneyama Mountain, Okayama City (34°5 ‘2″ ‘ N, 133º8 ‘8″ ‘E).
In both field experiments, the food (thawed commercially available cricket Gryllus bimaculatus, 2 g) was pinned to the leaves or stems of the shrubs inhabited by P. japonica with a metal paper clip according to the experimental method of Thornhill (1992). The food was placed from 9 to 10 am, and a video recording was started by a video camera (HDR-PJ590V, Sony, Tokyo), when an adult was found in or near the food for 5 h after the food was placed. In this study, 81 behaviors were observed using a pinned cricket.
The linear distances between the individuals who performed the wing-waving behavior and the other individual (who observed the wing-waving behavior) were measured. Each distance was measured by taping the ground every centimeter from the dead insects, and we recorded and determined the distances later in the video recording. The linear distance between individuals was measured by recording a video and then saving the video as an image using Image J (Ver.1.50i). In addition, when adults were found near dead insects in the field, the same recording and measuring procedures were used. A total of 18 behaviors were recorded in cases using naturally dead insects as a food resource.
When wing-waving behavior was observed, the following three parameters were recorded: (1) the number and duration of wing flashes, (2) the linear distance between the sender and receiver of wing flashes (measured from the sender’s head to the receiver's head), and (3) individual behaviors during wing-waving behavior, including courtship strategies, the winners and losers of male–male competitions, and satellite behaviors of loser males. Based on the recoded data, we calculated the frequency of wing-waving behaviors per minute, which was the number of wing flashes divided by the duration of the wing-waving behavior and then multiplied by 60. We compared the frequencies of wing-waving behaviors between two males at each satellite behavior by defeated males after courtship, before male–male competition, and after male–male competition.
Statistics
One-way analysis of variance (ANOVA) and Tukey’s HSD test were calculated using JMP version 12.2 (SAS Institute Inc., 2015). In addition, a generalized linear model (GLM) using logistic regression was used for statistical analysis of the winner or loser of male–male competition and the frequency of wing flashes. The significance level was set at P = 0.05 in each test.
Results
Description of wing-waving behavior
P. japonica mainly use wing-waving behavior for conspecific interactions. The wing-waving behaviors of P. japonica are similar to those of P. debilis (Magnier and Montgomery 2017). In detail, P. japonica lifts its forewings and hindwings from a stationary position so that they rotate the right wings would be going clockwise and the left counterclockwise when viewed P. japonica from the front. After raising the wings to an approximately 45° angle with the thorax as the axis, the wings are lowered vertically at about the same speed as they were raised, and the wings are returned to their resting positions. During this sequence of actions, the forewings and the hind wings are slightly separated, and the hind wings follow the forewings (Fig. 1, ESM S1). In this study, we defined this sequence of actions as a single wing-waving behavior act.
Laboratory experiments
Figure 5 shows the frequencies of wing-waving behaviors during courtship. Of 40 pairs observed, 31 courtships by males were observed, of which, 27 cases were confirmed to mate. Wing-waving behavior was confirmed in all 31 males who were observed courting, while only four females performed wing-waving behavior (Tukey HSD test, P < 0.001).
Field experiments
In the field experiments, 99 behaviors were observed. When we used a pinned cricket, 81 behaviors (nuptial gift: N = 26, male–male competition: N = 26, satelliting: N = 29) were observed. When a naturally dead insect was used as a food resource, a total of 18 behaviors (nuptial gift: N = 8, male–male competition: N = 5, satelliting: N = 5) were recorded.
Comparison of the frequency of wing-waving behavior between naturally dead insults and pinned crickets revealed no significant differences (Tukey HSD test, P = 0.0914). Therefore, in this study, both results were combined.
Wing-waving behavior was observed in the following three cases; (1) nuptial gifting (as courtship behavior), (2) male–male competition, and (3) satelliting behavior (loser male’s behavior after the male–male competition). In the present study, males holding food for nuptial gifting sometimes showed wing–waving behavior with attacks on houseflies (Muscidae) and ants (Formicidae) that came to the food (N = 3). In the present study, forced copulation without food during copulation (N = 1), and females finding the food before the male takes up the food (N = 2) were observed, but they were not included in the present analysis due to the small number of observations.
Figure 6 shows the average inter-individual distance during nuptial gifting (courtship behaviors), male–male competition, and satellite behavior. The wing-waving behavior was always observed when the distance between the two individuals was less than 15 cm (N = 99). Therefore, in the present study, the interactions were defined as a behavior performed with 15 cm or less between the two individuals. Comparison of average distances between the two individuals during each behavior showed no significant difference among the three cases (ANOVA, F2,94 = 0.3478, P = 0.707).
Since the average frequencies of wing-waving behavior did not differ at the two locations (river bank of Asahikawa: 0.53 ± 0.02, Tsuneyama mountain road: 0.56 ± 0.02, Tukey HSD test, P = 0.217), the data of both populations were calculated as one analysis.
Figure 7 shows the frequencies of wing- waving behavior for each behavior. In four of the 40 pairs, mating behavior was not observed. In the case of nuptial gifting, males performed wing-waving behavior toward females in all cases (N = 34), but only 3 out of 34 females showed wing-waving behavior toward males. Thus, the frequency of wing-waving behavior in females was significantly lower than that in males (Tukey HSD test, P < 0.001, Fig. 7A). Comparing the results of nuptial gifting in the field and laboratory experiments, there was no significant difference in the frequency of wing-waving behavior (Tukey HSD test, laboratory male vs. field male; P = 0.2954, laboratory female vs. field female; P = 0.9954). Before a male–male competition, both males defended their own food, and males without food attacked other males with food. In these cases, males always showed wing-waving behavior (Fig. 7B). There was no significant difference in the frequencies of wing-waving behaviors between the defender and the attacker (Tukey HSD test, P = 0.1289, Fig. 7B). Figure 7C shows a comparison of wing-waving behaviors between winner and loser (satellite) males after male–male competition. Winning males had significantly higher frequencies of wing-waving behavior than losing males (Tukey HSD test, P < 0.0001, Fig. 7C).
Frequencies of wing-waving behavior during each behavior; (A) comparison between males and females during nuptial gifting, (B) comparison between attackers and defenders during male–male competition, and (C) comparison between winner and loser (satellite) males after male–male competition. Significant differences were found in the intervals with different letters (Tukey HSD test, P = 0.05)
Figure 8 shows the frequencies of wing-waving behavior before each male–male competition by the winner and loser of the competition. The frequency of wing-waving behaviors by winner males was significantly higher than that by loser males (GLM, χ2 = 7.039, d.f. = 1, P = 0.008).
Discussion
Many species in the genus Panorpa show a mating system called resource-defense polygyny (Emlen and Oring 1997). That is, males compete with other males to defend resources, and also to monopolize opportunities to mate with scorpionfly females (Byers and Thornhill 1983; Magnier and Montgomery 2017). Panorpa japonica has also been confirmed to be resource-defense polygyny (Thornhill 1981, 1992). Magnier and Montgomery (2017) suggested that the wing-waving behavior of P. debilis, a closely related species of P. japonica, relates to resource-defense polygyny, and they predicted that the frequencies of wing-waving behavior are higher in males than females in P. debilis. In the present study, males holding food for nuptial gifting exhibited wing-waving behavior accompanied by attacks on houseflies (Muscidae) and ants (Formicidae) that came to the food (N = 3). Thus, similar to P. debilis (Magnier and Montgomery 2017), we showed the possibility of using wing-waving behavior in interspecific competition over the same food resource.
In the present study, courting males always showed wing-waving behavior (Fig. 7B), whereas females showed almost no wing-waving behavior toward males during nuptial gifting in P. japonica (Fig. 5, Fig. 7A). In the blowfly Chrysomya flavifrons, which is known for its complex courtship behavior, differences in courtship behavior between lab and field populations have been reported (Butterworth et al. 2019). However, the present results of the frequency of wing-waving behavior in P. japonica were not differed between the field and laboratory experiments. Therefore, the wing-waving behavior of P. japonica was not affected by the external environment. In addition, a few cases of female-to-female wing-waving behavior were observed (N = 2). These show sexual dimorphisms in the frequencies of wing-waving behavior in P. japonica. It is known that P. japonica uses three mating tactics: forced mating, nuptial gifts, and feeding mating (Byers and Thornhill 1983; Thornhill 1992a, Sato and Fujiyama 2018, Ishihara and Miyatake 2020). The present results show males performed wing-waving behavior toward females, but it is unclear whether females have wing-waving behavior in the three mating tactics in P. japonica.
Magnier and Montgomery (2017) showed that both males and females in P. debilis performed wing-waving behavior when the individuals of the same species approached them, and thus they considered that wing-waving behavior may be a form of communication with the same species or competition for food resources against other species. On the other hand, in P. japonica, the frequency of wing-waving behavior was higher in males than in females, indicating that the wing-waving behavior likely functions as a part of sexual selection, at least in the case of P. japonica.
In the male–male competitions of P. japonica, winner males performed significantly more wing-waving behavior than loser males (Fig. 8). In addition, winner males showed more wing-waving behavior toward satellite (or loser) males in all cases (N = 34), and wing-waving behavior was observed by only three satellite males. Therefore, we consider that the male wing-waving behavior of P. japonica is a display in male–male competition over food resources. In the future, it would be beneficial to clarify whether females of P. japonica show wing-waving behavior while eating food against conspecific females visiting the food.
It has been suggested that wing-waving behavior is performed by North American scorpionflies before male–male competition (Thornhill 1981). However, no studies have observed the wing-waving behavior during an incident of male–male competition. Thus, the present result is the first report to suggest that the winner of a male–male competition used wing-waving behavior as a display, probably, against the loser during male–male competition.
In resource-defense polygyny, the ability to acquire resources and defend resources from other males depends on the qualities of the male, such as strength, in male–male competition (Thornhill 1981). Studies of European Panorpidae found a significant correlation between male health and strength in male–male competition (Sauer et al. 1998), and this suggests strength in male–male competitions could be related to the larval growth environment (Thornhill and Sauer 1992). In P. japonica, it has been suggested that fluctuating asymmetry of forewing (FA) may increase with exposure to environmental stresses such as poor nutrition and parasites during the larval stage (Thornhill 1992). If wing-waving behavior before male–male competition acts as a signal to indicate the male’s condition, males with infrequent wing-waving behavior may give up on male–male competition and escape. In the future, it would be good to show the relationships between the significance of signals in male–male competition and aspects of the larval growth environment such as nutritional status.
There was a sexual difference in the frequency of wing-waving behavior between P. debilis and P. japonica. It is known that there are interspecific differences in the area of the black portion of the wings of Panorpa species (e.g., Hartbauer et al. 2015), and the use of wing-waving behavior may aid in species recognition. Also, as in the case of the blowflies (e.g., Eichorn et al. 2017, Butterworth et al. 2020), there may be a sex recognition system in P. japonica based on the light reflected from the wings. In the future, it is necessary to verify whether there are differences between males and females in wing patterns and reflected light generated during wing-waving behavior in P. japonica. The mating tactics and patterns even in the same family, the Panorpidae, may vary from species to species (e.g., Byers and Thornhill 1983).
In the present study, however, we observed few satellite behaviors of males in which the loser males attempted to force mating with females. Satellite males frequently tried to re-enter the feeding arena, the place for male–male competition. Then, the winner male attacked the satellite male. However, the satellite males always escaped without counter-attacking the winner male. In addition, satellite males in the present study invaded the feeding arena and released pheromones while winner males were mating with the female. Similar pheromone-releasing behavior has been observed by Thornhill (1992a). In a study on P. japonica by Thornhill (1992b), loser males were satellites around the food, and they attempted forced mating with females. On the other hand, in the present study, satellite loser males did not attempt forced mating. Why the results differ for the same species will be an interesting issue to study. Thornhill (1992b) observed mating behavior using a population of P. japonica in Aichi, Japan, but we used populations in Okayama in western Japan. Behavior may vary among local populations due to factors such as differences in climate and predators. In the future, it will be required to compare male mating tactics and wing-waving behavior using multiple species of Panorpidae and different regional populations.
Change history
06 December 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10164-021-00733-y
References
Andersson M (1994) Sexual selection. Princeton University Press
Benelli G, Ricciardi R, Romano D, Cosci F, Stefanini C, Lucchi A (2019) Wing-fanning frequency as a releaser boosting male mating success – High-speed video analysis of courtship behavior in Campoplex capitator, a parasitoid of Lobesia botrana. Insect Sci. https://doi.org/10.1111/1744-7917.12740
Butterworth NJ, Byrne PG, Wallman JF (2019) The blowfly waltz: field and laboratory observations of novel and complex dipteran courtship behavior. J Insect Behav 32:109–119
Butterworth NJ, White TE, Byrne PG, Wallman JF (2020) Love at first flight: wing interference patterns are species-specific and sexually dimorphic in blowflies (Diptera: Calliphoridae). J Evol Biol 34:558–570
Byers GW, Thornhill R (1983) Biology of the Mecoptera. Annu Rev Entomol 28:203–228
Candolin U, Reynolds JD (2001) Sexual signaling in the European bitterling: females learn the truth by direct inspection of the resource. Behav Ecol 12:407–411
Cowling DE, Burnet B (1981) Courtship songs and genetic control of their acoustic characteristics in sibling species of the Drosophila melanogaster subgroup. Anim Behav 29:924–935
Emlen ST, Oring LW (1997) Ecology, sexual selection, and the evolution of mating systems. Science 197:215–223
Hartbauer M, Gepp J, Hinteregger K, Koblmüller S (2015) Diversity of wing patterns and abdomen-generated substrate sounds in 3 European scorpionfly species. J Insect Sci 22:521–531
Hilton DFJ (1986) A survey of some Odonata for ultraviolet patterns. Odonatologica 15:335–345
Ishihara R, Miyatake T (2020) Relationships between mating tactics and male traits such as body size and fluctuating asymmetry in the Japanese scorpionfly. J Ethol 38:233–239
Kurokawa T, Sato K, Fujiyama S (2012) Daily photoperiod and mating tactics of Panorpa (Panorpidae) species and Panorpodes paradoxus (Panorpidae). Faculty Sci Shinshu Univ 34:100–105
Madeline BG, Damian OE, Michael MK (2015) Female preference for multi-modal courtship: multiple signals are important for male mating success in peacock spiders. Proc R Soc B 282:1820
Magnier B, Montgomery G (2017) Novel wing-flashing behavior in a scorpionfly(Panorpa debilis) May be competitive. J Insect Behav 30:247–258
Miller CW, Svensson EI (2014) Sexual selection in complex environments. Ann Rev Entomol 59:427–445
Prokopy RJ, Bush GL (1973) Mating behavior of rhagoletis pomonella (Diptera: Tephritidae): IV. Courtship Can Entomol 105:873–891
Rupprecht R (1974) Vibration signals during copulation of Panorpa (Mecoptera, Insecta). Experientia 30:340–341
Sato K, Fujiyama S (2018) Comparison of mating behavior of nine species in Japanese scorpionfly -special references to the behavior of nuptial gift-. Ann Environ Sci Shinshu Univ 40:64–71 (in Japanese)
Sauer KP, Lubjuhn T, Sindern J, Kullmann H, Kurtz J (1998) Mating system and sexual selection in the scorpionfly Panorpa vulgaris (Mecoptera: Panorpidae). Naturwissenschaften 85:219–228
Scholes E (2008) Evolution of the courtship phenotype in the birds of paradise genus Parotia (Aves: Paradisaedae): homology, phylogeny, and modularity. Biol J Linn Soc 94:491–504
Schultz TD, Fincke OM (2009) Structural colours create a flashing cue for sexual recognition and male quality in a Neotropical giant damselfly. Funct Ecol 23:724–732
Setoguchi S, Takamori H, Aotsuka T, Sese J, Ishikawa Y, Matsuo T (2014) Sexual dimorphism and courtship behavior in Drosophila prolongata. J Ethol 32:91–102
Sweeney A, Jiggins C, Johnsen S (2003) Polarized light as a butterfly mating signal. Nature 423:30–31
Takeuchi T (2017) Agonistic display or courtship behavior? A review of contests over mating opportunity in butterflies. J Ethol 35:3–12
Thornhill R (1981) Panorpa (Mecoptera: Panorpidae) scorpionflies: systems for understanding resource-defense polygyny and alternative male reproductive efforts. Annu Rev Ecol Syst 12:355–386
Thornhill R (1992a) Female preference for the pheromone of males with low fluctuating asymmetry in the Japanese scorpionfly (Panorpa japonica: Mecoptera). Behav Ecol 3:277–283
Thornhill R (1992b) Fluctuating asymmetry and the mating system of the Japanese scorpionfly, Panorpa japonica. Anim Behav 44:867–879
Thornhill R, Alcock J (1983) The evolution of insect mating systems. Harvard University Press
Thornhill R, Sauer KP (1992) Genetic sire effects on the fighting ability of sons and daughters and mating success of sons in a scorpionfly. Anim Behav 43:255–264
Tsutsumi T (2010) Scorpionfly fauna of Fukushima prefectural forest “Forest Park Adarata.” Ann Rep Synthetic Study Fukus Univ Nat Hum 8:8–17
Acknowledgements
This work was supported by a grant from the Japan Society for the Promotion of Science (JSPS), KAKENHI 18H02510 and 21H021568 to TM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declares that he/she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to a retrospective Open Access order.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 12012 KB)
Supplementary file2 (MP4 26993 KB)
Supplementary file3 (MP4 5764 KB)
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Ishihara, R., Miyatake, T. Wing-waving behaviors are used for conspecific display in the Japanese scorpionfly, Panorpa japonica. J Ethol 39, 267–274 (2021). https://doi.org/10.1007/s10164-021-00709-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-021-00709-y