Abstract
We described and compared the mating behavior and morphology of the reproductive system in chromodorid nudibranchs, then, examined how extensively the unique usage of the penis (autotomy and sperm removal) evolved among Chromodorids. In addition to Ardeadoris egretta, all of examined five species in Glossodoris autotomized their penises at the last stage of copulation. An interspecific difference was seen in penis autotomy in Noumea and Goniobranchus. A spiral structure was recognized in the vas deferens of autotomized species. This structure is supposed to be undifferentiated “next penises” stored in the vas deferens, which ensure successive copulation in autotomized species. Though the basic mechanism of penis replenishment was consistent, the reason to autotomize their penises may not be the same. Goniobranchus reticulatus is reported to remove sperm already stored in the mating partner’s sperm storage organ(s) with backward-pointing spines on the surface of its penis. Contrary to G. reticulatus, all of ten species that autotomized their penises in the present study did not have thorny but smooth penises. When they autotomized their penises, the tip of the penises still remained in the vagina of the partners. This suggests that autotomized penises in these nudibranchs function as a kind of copulatory plugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sperm displacement, in which allosperm from previously copulated males already stored in the copulatory pouch of mating partners is removed, has been reported in various animals since first discovered in a damselfly (Waage 1979) and recognized as a typical example of the intensity of sperm competition (Córdoba-Aguilar et al. 2003). Among mollusks, sperm displacement was clarified in a cuttlefish (Wada et al. 2005) and a nudibranch (Sekizawa et al. 2013, 2019). The latter instance proved that sexual selection functions strongly even in simultaneous hermaphrodites like nudibranchs. Though the effectiveness of removing the sperm of competing males is the same in all animals (Simmons and Siva-Jothy 1998), the method of removal varies in each animal. For example, dragonflies use a hook-shaped structure located at the tip of the male copulatory organ, cuttlefish use an arm (arm III), and a nudibranch, Goniobranchus reticulatus (as Chromodoris reticulata in Sekizawa et al. 2013), uses its spiny penis. Furthermore, the nudibranch autotomized its penis after every copulation and replenished it within about 24 h to perform another copulation (Sekizawa et al. 2013).
It has been reported the morphology of internal reproductive organs and the penis morphology among nudibranchs are extremely diversified (Valdes et al. 2010). Furthermore, there have been very few detailed studies on the relationship between mating behavior and the morphology of reproductive organs. Whether penis autotomy and its replenishment, and sperm replacement are generally developed among nudibranchs, especially Chromodorids, or specific to an exceptional species is not known. Sekizawa et al. 2018 clarified Goniobranchus tinctorium (as Chromodoris tinctoria in Sekizawa et al. 2018), a closely related species of G. reticulatus (as Chromodoris reticulata in Sekizawa et al. 2018), did not autotomize its penis after copulation nor remove allo-sperm with the penis. Though G. tinctorium was also able to replenish its penis, it needed fairly long period of time, 27 days in a case.
In this study, we will describe and compare the mating behavior and morphology of the reproductive system in chromodorids. Then, we will examine how extensively the unique usage of the penis (autotomy and sperm removal) evolved among chromodorids.
Materials and methods
Study animals
We studied the following 19 Chromodorididae species: Cadlinella ornatissima (Risbec 1928), Chromodoris colemani (Rudman 1982), Goniobranchus rufomaculatus (Pease 1871), Goniobranchus coi (Risbec 1956), Goniobranchus geometricus (Risbec 1928), Goniobranchus decorus (Pease 1860), Noumea norba (Marcus and Marcus, 1970), Noumea angustolutea (Rudman 1990), Noumea crocea (Rudman 1986), Hypselodoris maculosa (Pease 1871), Hypselodoris infucata (Ruppell and Leuckart 1831), Hypselodoris bullocki (Collingwood 1881), Risbecia tryoni (Garrett 1873), Glossodoris atromarginata (Cuvier 1804), Glossodoris hikuerensis (Pruvot-Fol 1954), Glossodoris rufomarginata (Bergh 1890), Glossodoris sp. cf. pallida (Ruppell and Leuckart 1828), Glossodoris sp. cf. cincta (Bergh 1888) and Ardeadoris egretta (Rudman 1984). We collected most of the animals using SCUBA from shallow coral reefs (depth of 1–4 m depth) near the Sesoko Station of the University of the Ryukyus, Okinawa, Japan (26°38′N, 127°52′E), during their reproductive season (April to June) in 2013. We also collected several individuals of Glossodoris rufomarginata from the tidal zone on Isshiki beach, Kanagawa, Japan (35°27′N, 139°57′E). In Japan, these nudibranchs commonly occur on shallow rocky coasts from central to southern Honshu and around the Ryukyu Islands (Nakano 2004, 2018; Ono 2004). We referred Gosliner et al. (2015, 2018), Nakano (2018) and Soong et al. (2020) for identification.
Maintenance
The collected animals were maintained and identified individually in perforated small plastic cages submerged in a seawater tank. The tank was submerged in running seawater and aerated. We did not feed the sea slugs, since their food consumption decreases substantially during their reproductive season (Thompson 1966).
Observations of mating behavior
We used a clear, acrylic framed experimental tank measuring 10 × 15.5 × 5 cm (length, width, height; 775 mL). We placed two individuals into the experimental tank and measured their body length (from the anterior end to the posterior end of the mantle), while they were slowly crawling. Then we visually observed the sequence of their mating behavior (pre-copulatory behavior, during copulation, and post-copulatory behavior), and timed the duration of copulation (from the contact of copulatory apparatus to separation). Individuals used in mating observations were kept together for 6 h after the first copulation to verify if they could copulate repeatedly. In the case that they did not try the second copulation within 6 h, we separated them and placed them together again after 24 h. We stopped observing the mating behavior when the animals continued copulating for more than 3 h.
Anatomical observations of reproductive organs
Following behavioral observations, we fixed individuals of Noumea norba, N. crocea, Glossodoris sp.cf. cincta, G. sp. cf. pallida, G. atromarginata, G. hikuerensis, G. rufomarginata, Goniobranchus coi, Chromodoris colmani and Ardeadoris egretta in 70% ethanol overnight. We dissected out the reproductive organs and anatomically observed their morphology. Then, we dehydrated and cleared the male reproductive ducts with methyl salicylate to precisely observe them using a stereomicroscope.
Results
Mating behavior
Cadlinella ornatissima
We observed copulation twice among five individuals (TL 16.42 ± 2.05 mm). Copulation began with reciprocal touching of the right side of the bodies. They then adjusted the tip of the projected genitalia of the other, and each inserted their penises into the partner’s vagina. The copulation lasted more than 3 h in both cases. Though they usually moved with their tentacles vigorously shaking, they stopped shaking them during copulation. At the beginning of copulation, they tightly put their mantles together and their copulatory apparatuses were hardly seen under the mantles. Two hours later, they gradually separated, and the copulatory apparatuses were exposed. They never stretched nor autotomized the penises.
Noumea norba
We observed copulation twice among three individuals (TL 18.57 ± 1.81 mm) under a stereomicroscope. The durations of copulation were 5 min 1 s and 22 min 49 s. At the end of copulation, the two individuals moved away and their penises gradually stretched. Finally, the penises snapped off spontaneously and copulation ended.
Noumea angustolutea
We observed copulation twice between two individuals (TL 9.7 mm, 13.3 mm) under a stereomicroscope. The durations of copulation were 1 h 32 min 7 s and 2 h 6 min 33 s. At the end of copulation, the two individuals moved away in opposite directions and their penises gradually stretched. When their penises snapped off, the penis of mating partner was still inserted. They crawled for a while dragging the other’s penis.
Noumea crocea
We observed copulation six times among four individuals (TL 16.15 ± 5.16 mm). The durations of copulation were 7 min 2 s, 9 min 11 s, 21 min 31 s, 22 min 30 s, and 37 min 1 s (average 19 min 27 s). In the remaining one case, they continued copulation for more than 3 h. They never stretched their penises and could copulate repeatedly within a relatively short period of time.
Chromodoris colemani
We observed copulation twice among seven individuals (TL 37.19 ± 4.18 mm). The duration of copulation was 57 s and 2 min 27 s. During copulation, both individuals stayed motionless. Copulation ended when either individual bit the other with its buccal mass and separated the tip of their copulatory apparatus. They did not autotomize their penises.
Goniobranchus coi
We observed copulation three times among five individuals (TL 59.88 ± 4.48 mm). The durations of copulation were 20 min 53 s, 23 min 40 s, and 33 min 56 s. Though they fluttered the mantle when they were crawling, they did not move it during copulation. At the end of copulation, mating partners slowly moved forward in opposite directions, each penis was still inserted in the other. Then either individual pulled its penis out and the other followed. They autotomized their penises after both of them had finished pulling out. In contrast to G. reticulatus, which took 20.57 ± 7.04 min to autotomize the penis after the end of copulation (Sekizawa et al. 2013), G. coi autotomized immediately. This difference is supposed to depend on the penis thickness of two species.
Goniobranchus geometricus
We observed copulation twice among three individuals (TL 22.97 ± 4.86 mm). The duration of copulation was 18 min 47 s and 22 min 54 s. They frequently fluttered the head part of the mantle, similar to that observed in G. coi, when they were crawling. At the end of copulation, either individual would leave the other, stretching its penis. After finally removing the penis, it was autotomized.
Goniobranchus rufomaculatus
We observed copulation twice in random combination of four individuals (TL 24.28 ± 1.40 mm). The copulation lasted more than 3 h in both cases. They were basically motionless during copulation. They did not autotomize their penises.
Goniobranchus decorus
We observed copulation twice among three individuals (TL 10.80 ± 2.94 mm). The duration of copulation was 28 min 8 s and 1 h 8 min 25 s. They did not autotomize their penises.
Hypselodoris maculosa
We observed copulation twice among three individuals (TL 10.80 ± 2.94 mm). The copulation lasted for more than 3 h in both cases. They stayed almost motionless during copulation and did not autotomize their penises.
Hypselodoris infucata
We observed copulation four times among four individuals (TL 34.55 ± 2.25 mm). The duration of copulation was 25 min 5 s, 28 min 59 s, 50 min 17 s, and more than 3 h in one case. They rotated clockwise slowly during copulation and turned about 90° in 30 min. They did not autotomize their penises.
Hypselodoris bullocki
We observed copulation twice among five individuals (TL: 34.24 ± 3.98 mm). The copulation lasted 2 h 27 min 46 s in one case and for more than 3 h in the other case. They did not autotomize their penises.
Risbecia tryoni
We observed copulation twice among three individuals (TL 67.30 ± 2.59 mm). The duration of copulation was 23 min 2 s and 41 min 32 s. When they were crawling, they fluttered the head part of their mantle, similar to that observed in G. geometricus and G. coi. They stopped fluttering it during copulation. At the end of copulation, either individual retracted its copulating apparatus and left the other. The other began to pursue, exposing its copulating apparatus. Neither individual autotomized their penis. When the front one stopped, the back one also stopped. The back one resumed their pursuit when the front one started to move again. The back one did not try to overtake or copulate with the front one. About 30 min after the beginning of its pursuit, the back one discontinued and left the front one. In this case, pursuing was post-copulatory behavior, but not pre-copulatory behavior. Though pursuing behavior in the wild has been often observed and roughly mentioned in several books, it has not been studied specifically and its purpose is still unknown (Behrens 2005; Nakano 2004, 2018; Ono 2004).
Glossodoris sp. cf. cincta
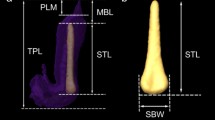
We observed copulation nine times among 19 individuals (TL 32.2 ± 12.8 mm). The average duration of copulation was 41 min 29 s ± 20 min 24 s. They did not move during copulation. They left each other at the last stage of copulation and stretched their penises for longer than observed in the other five congeneric species. When they finally autotomized their penises, the tip still remained in the vagina of their partners. We certified penis autotomy in all of the nine observed instances. The length of autotomized penises was about 20 mm and the surfaces appeared smooth (Fig. 1a, b). In two out of the nine copulations, they could recopulate within a relatively short period of time, 30 min after the first copulation in one case and 6 h in the other.
Glossodoris atromarginata
We observed copulation four times in random combination of eight individuals (TL 29.9 ± 6.1 mm). The average duration of copulation was 13 min 35 s ± 2 min 13 s. They did not move during copulation. They left each other in opposite directions at the last stage of copulation. They stretched their penises and autotomized them, leaving the tip in the vagina of the partner. We certified the autotomy of very thin penises in all of four observed instances. The surfaces of the autotomized penises were smooth. Though none of four copulated individuals could recopulate within a short period of time, all of them could after 24 h or longer.
Glossodoris hikuerensis
We observed copulation eight times between two individuals (TL 53.4 ± 16.0 mm). The average duration of copulation was 16 min 26 s ± 6 min 24 s. They stayed almost motionless just before, during, and after copulation. They did not strongly pull their penises from each other after copulation. When they autotomized their penises, the tip remained in the vagina of the partners. We certified penis autotomy in all of the eight observed instances. The autotomized penises were very thin, rather short, and their surfaces were smooth without spines. We certified successive copulations within a fairly short period of time in two cases. They were able to recopulate 1 h after the first copulation in one case, and in another case for 2 h and 4 h in their second and third copulation, respectively.
Glossodoris rufomarginata
We observed copulation five times among five individuals (TL 23.9 ± 9.9 mm). The average duration of copulation was 12 min 3 s ± 5 min 45 s. They stopped moving when they began to copulate. They left each other in opposite directions at the last stage of copulation. They stretched their penises and autotomized them, leaving the tip in the vagina of the partner. We certified penis autotomy in four out of five of the observed instances (the remaining one case was uncertain). The autotomized penises were too thin to collect. Their surfaces seemed smooth when stretched. They were able to copulate successively in one case, in which the second copulation occurred 1.5 h after the first one.
Glossodoris sp. cf. pallida
We observed copulation five times among four individuals (TL 35.9 ± 16.1 mm). The average duration of copulation was 34 min 41 s ± 14 min 3 s. They stretched their penises and autotomized them, leaving the tip in the vagina of the partner. We certified penis autotomy in all of the five observed instances. The surfaces of autotomized penises were smooth without spines. Though none of the four copulated individuals could recopulate repeatedly within a short period of time, all of them were able to do so 24 h or longer after the first copulation.
Ardeadoris egretta
We observed copulation four times among six individuals (TL 68.3 ± 5.7 mm). The average duration of copulation was 38 min 11 s ± 21 min 47 s. They stretched their penises and autotomized them, leaving the tip in the vagina of the partner. We certified penis autotomy in all of the four observed instances. Most of the autotomized penises were 10 mm or longer with smooth surfaces (Fig. 1c, d). Though none of six copulated individuals could recopulate repeatedly within a short period of time, all of them were able to do so 24 h or longer after the first copulation.
Morphology of the reproductive system
We observed precisely morphology of the reproductive system of Noumea, Glossodoris and Ardeadoris that included penis-autotomy species.
Noumea norba
The relative size and arrangement of each reproductive organ (Fig. 2) were almost the same as those schematically shown in Rudman (1984: Fig. 101F). A spiral structure (Fig. 6 d) was seen in one cleared sample out of the two.
Noumea crocea
The seminal receptacle was larger than those generally seen in this genus (Rudman 1984). The copulatory pouch was also larger than that of N. norba. This morphology is more similar to that of Goniobranchus instead of Noumea (Fig. 2). A spiral structure (Fig. 6e) was not seen in the vas deferens of the two dissected individuals.
Glossodoris sp. cf. cincta
The relative size and arrangement of each reproductive organ, copulatory pouch, seminal receptacle, prostate gland, vas deferens, vaginal canal, and seminal reservoir was almost the same as those schematically shown in Rudman (1984: Fig. 101E). The vas deferens was relatively long and thick, and the vaginal canal was very long compared to that in congeneric species (Fig. 3). A long spiral structure (Fig. 6f, g) was seen in the vas deferens of all four observed samples.
Glossodoris atromarginata
The relative size and arrangement of each reproductive organ (Fig. 4) was almost the same as those schematically shown in Rudman (1984: Fig. 101E). A short spiral structure (Fig. 6 h) was seen in three out of the seven observed cleared samples.
Glossodoris hikuerensis
The relative size and arrangement of each reproductive organ were almost the same as those schematically shown in Rudman (1984: Fig. 101E). The vas deferens and the vaginal canal were very short and had a simple structure (Fig. 4). The spiral structure (Fig. 6i) seen in the vas deferens was as long as that of G. sp.cf. cincta.
Glossodoris rufomarginata
The relative size and arrangement of each reproductive organ (Fig. 4) were almost the same as those schematically shown in Rudman (1984: Fig. 101E). The vas deferens and the vaginal canal were very short and had a simple structure. A successive long spiral structure (Fig. 6j) was seen in the vas deferens of all three cleared samples observed.
Glossodoris sp. cf. pallida
The copulatory pouch and the seminal receptacle were larger than those shown in Rudman (1984: Fig. 101E). The vas deferens was long, thick, and muscular, and the prostate gland was very long (Fig. 5). A short spiral structure (Fig. 6 k) was seen in the vas deferens.
Vas deferens cleared using methyl salicylate of (a) Chromodoris colemani, (b) Goniobranchus coi, (c) G. orientalis, (d) Noumea norba, (e) N. crocea, (f), (g) Glossodoris sp. cf. cincta, (h) G. atromarginata, (i) G. hikuerensis, (j) G. rufomarginata, (k) G. sp. cf. pallida, (l) Ardeadoris egretta. (a)–(c) 500 μm, (d)–(g) 100 μm, (h)–(l) 250 μm
Ardeadoris egretta
Though this is the first report on the internal reproductive morphology in this species, the relative size and arrangement of each reproductive organ (Fig. 5) was almost the same as those of Glossodoris spp., schematically shown in Rudman (1984: Fig. 101E). The distal to central part of the vaginal canal was slightly thickened. A long spiral structure (Fig. 6l) was seen in the vas deferens.
Discussion
The ability to autotomize and replenish the penis after every copulation differed among the 19 examined species. All six species in Glossdoris and Ardeadoris egretta autotomized, but Cadlinella ornatissima, all three species in Hypselodoris, Chromodoris colemani, and Risbecia tryoni did not. In Noumea, N. norba and N. angustolutea did and N. crocea did not. Additionally, in Goniobranchus, G. coi and G. geometricus did and G. rufomaculatus and G. decorus did not. Interspecific difference in this ability in Goniobranchus was reported between two related species (Sekizawa et al. 2018). This study supports that conclusion and expands the range of applicability. We recognized a spiral structure in the vas deferens of those species that had an ability to autotomize their penises in Noumea, Goniobranchus, Glossdoris, and Ardeadoris. Spiral structures are supposed to be undifferentiated “next penises” (Sekizawa et al. 2013) stored in the vas deferens, which ensure successive copulations. The basic mechanism of penile replenishment seemed consistent in the examined species.
Additionally, there was a large interspecific difference in the length of time required to be able to copulate again after the autotomy of the penis. Glossodoris spp., G. sp. cf. cincta, G. hikuerensis, and G. rufomarginata were able to copulate successively within a few hours (repeatable species), while G. atromarginata, G. sp. cf. pallida, and A. egretta could not copulate again within 24 h (non-repeatable species) of the first copulation, as reported in Goniobranchus reticulatus (as Chromodoris reticulata in Sekizawa et al. 2013). This difference in required time is related to the morphological difference in the spiral structure in the vas deferens of each species. While the spiral structures were very long and whirling in repeatable species, they were short and simply winding in non-repeatable species. In contrast, the structure or the length of male reproductive system itself was not related to the required time. The vas deferens and the prostatic gland in G. sp. cf. cincta (repeatable), G. rufomarginata (repeatable), G. atromarginata (non-repeatable), and G. sp. cf. pallida (non-repeatable) were relatively long and had a complicated structure, while those in G. hikuerensis (repeatable) and A. egretta (non-repeatable) were relatively short and simple (Table 1). The arrangement and the morphology of the reproductive system in A. egretta was most similar to that in G. hikuerensis.
Though the basic mechanism of penile replenishment was consistent in examined species, the reason for autotomization seemed to differ among them. Goniobranchus reticulatus was reported to remove sperm already stored in a mating partner’s sperm storage organ(s) with backward-pointing spines on the surface of its penis (Sekizawa et al. 2013, 2019). They autotomized their penises after pulling out from the vagina of the mating partner. Contrary to G. reticulatus, all 10 species that autotomized their penises in this study, G. coi, G. geometricus, N. norba, N. angustolutea, A. egretta, and all five Glossodoris spp. did not have thorny penises; instead the surfaces of their penises appeared smooth. When they autotomized their penises, the tip remained in the vagina of the partners. This suggests that autotomized penises in these nudibranchs do not work as sperm removers, but as kind of copulatory plugs.
Change history
14 December 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10164-021-00736-9
References
Behrens DW (2005) Nudibranch behavior. New World Publications Inc., Jacksonville, USA
Córdoba-Aguilar A, Uhía E, Rivera AC (2003) Sperm competition in Odonata (Insecta): the evolution of female sperm storage and rivals’ sperm displacement. J Zool 261:381–398
Gosliner TM, Valdes A, Behrens DW (2015) Nudibranch & Sea Slug Identification Indo-Pacific. 408 pp. New World Publications Inc., Jacksonville, USA.
Gosliner TM, Valdés A, Behrens DW (2018) Nudibranch & sea slug identification: Indo-Pacific. New World Publications, Jacksonville
Nakano R (2004) Opisthobranchs of Japan Islands. (in Japanese). 304 pp. Rutles, Inc, Tokyo.
Nakano R (2018) Field Guide to Sea Slugs and Nudibranchs of Japan. (in Japanese). 544 pp. Bun-ichi co., Ltd. Tokyo.
Ono A (2004) Opisthobranchs of Ryukyu Islands. (in Japanese). 304 pp. Rutles, Inc., Tokyo.
Rudman WB (1984) The Chrornodorididae (Opisthobranchia: Mollusca) of the Indo-West Pacific: a review of the genera. Zool J Linn Soc 81:115–273
Sekizawa A, Seki S, Tokuzato M, Shiga S, Nakashima Y (2013) Disposable penis and its replenishment in a simultaneous hermaphrodite. Biol Lett 9: DOI: https://doi.org/10.1098/rsbl.2012.1150
Sekizawa A, Yama R, Nakashima Y (2018) Biological differences between Chromodoris reticulata and Chromodoris tinctoria (Opisthobranchia: Nudibranchia). Venus 76(1–4):45–52
Sekizawa A, Goto SG, Nakashima Y (2019) A nudibranch removes rival sperm with a disposable spiny penis. J Ethol 37(1):21–29. https://doi.org/10.1007/s10164-018-0562-z
Simmons LW, Siva-Jothy MT (1998) Sperm competition in insects: mechanisms and the potential for selection. In: Birkhead TR, Møller AP (eds) Sperm competition and sexual selection. Academic Press, London, pp 341–434
Soong GY, Wilson NG, Reimer JD (2020) A species complex within the red-reticulate Goniobranchus Pease, 1866 (Nudibranchia: Doridina: Chromodorididae). Marine Biodiversity (2020) 50: 25 https://doi.org/https://doi.org/10.1007/s12526-020-01048-w
Thompson TE (1966) Studies on the reproduction of Archidoris pseudoargus (Rapp) (Gastropoda Opisthobranchia). Philosophical Trans R Soc Ser B 250:343–374
Valdes A, Gosliner T, Ghiselin M (2010) Opisthobranchs. In: Leonard J, Cordoba-Aguilar A (eds) The Evolution of Primary Sexual Characters in Animals, Oxford University Press Inc. pp.200-300
Waage JK (1979) Dual function of the damselfly penis: sperm removal and transfer. Science 203:916–918
Wada T, Takegaki T, Mori T, Natsukari Y (2005) Sperm displacement behavior of the cuttlefish Sepia esculenta (Cephalopoda: Sepiidae). J Ethol 23(2):85–92
Yama R (2012) Studies on mating behavior and the structure of reproductive system in nudibranchs. (in Japanese). Graduation thesis of the laboratory of physiology in marine animals, Faculty of Bioresorces, Nihon University
Acknowledgements
We thank K. Sakai and other members of the Sesoko Station of the University of the Ryukyus for their help during the field survey in Okinawa. We appreciate K. Asahina, M. Suzuki, R. Yama, D. Murayama, and K. Kosoba from the College of Bioresource Sciences, Nihon University, for their advice and encouragement. This study was supported by JSPS Grant-in-Aid (no. 22570029) for Scientific Research to Y.N.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent
Irrelevant.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to a retrospective Open Access order.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Sekizawa, A., Tsurumi, Y., Ito, N. et al. Another usage of autotomized penis. J Ethol 39, 319–328 (2021). https://doi.org/10.1007/s10164-021-00706-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-021-00706-1