Abstract
The increased interest in the application of lasers in neuro-oncology prompted us to present our experience of using the laser technologies in the treatment of cerebral gliomas. The aim of the study was to evaluate the clinical efficacy of image-guided laser surface thermal therapy (LSTT) and its influence on survival of patients with glioblastoma (GBM).
Data of 91 patients (49 males, 42 females, mean age 51.4 years, range 23–70 years) with supratentorial GBMs located in close vicinity to or within the eloquent brain areas were retrospectively analyzed.
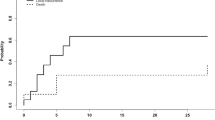
All patients were divided into two groups: LSTT group (n = 28) and control group (n = 63). There were no significant differences by gender, age, Karnofsky Performance Scale (KPS) score, and tumor location between groups. Total removal in the LSTT group was performed in 67.9%, in the control group—31.7% (p < 0.01); on the contrary, subtotal removal prevailed in the control group—52.4%; in the LSTT group, it was 32.1%. In postoperative period, there was no significant difference in KPS score between the groups (p = 0.89). A higher degree of resection provided an increase in survival rates (p < 0.01). The median overall survival was 15.5 ± 10.5 months, in the LSTT group 18.4 ± 11.7 and in the control group 14.3 ± 9.1 (p = 0.03). The application of image-guided LSTT in patients with GBMs of eloquent brain areas allowed the high rate of complete resection and improved overall survival without the negative effect on the functional status after surgery.


Similar content being viewed by others
References
Burke L, Rovin R, Cerullo L, Brown J (1985) Thermal effects of the Nd: YAG and carbon dioxide lasers on the central nervous system. Lasers Surg Med 5(1):67–71
Delgado-López P, Corrales-García E (2016) Survival in glioblastoma: a review on the impact of treatment modalities. Clin Transl Oncol 18(11):1062–1071. https://doi.org/10.1007/s12094-016-1497-x
Eyüpoglu I, Hore N, Savaskan N, Grummich P, Roessler K, Buchfelder M, Ganslandt O (2012) Improving the extent of malignant glioma resection by dual intraoperative visualization approach. PLoS One 7(9):e44885
Filippini G, Falcone C, Boiardi A et al (2008) Prognostic factors for survival in 676 consecutive patients with newly diagnosed primary glioblastoma. Neuro-Oncology 10(1):79–87
Gil-Robles S, Duffau H (2010) Surgical management of World Health Organization Grade II gliomas in eloquent areas: the necessity of preserving a margin around functional structures. Neurosurg Focus 28(2):E8. https://doi.org/10.3171/2009.12.FOCUS09236
González-Darder J, González-López P, Talamantes F, Quilis V, Cortés V, García-March G, Roldán P (2010) Multimodal navigation in the functional microsurgical resection of intrinsic brain tumors located in eloquent motor areas: role of tractography. Neurosurg Focus 28(2):E5. https://doi.org/10.3171/2009.11.FOCUS09234
Hawasli A, Kim A, Dunn G, Tran D, Leuthardt E (2014) Stereotactic laser ablation of high-grade gliomas. Neurosurg Focus 37(6):E1. https://doi.org/10.3171/2014.9.FOCUS14471
Hervey-Jumper S, Berger M (2014) Role of surgical resection in low- and high-grade gliomas. Curr Treat Options Neurol 16(4):284. https://doi.org/10.1007/s11940-014-0284-7
Jethwa P, Barrese J, Gowda A, Shetty A, Danish S (2012) Magnetic resonance thermometry-guided laser-induced thermal therapy for intracranial neoplasms. Oper Neurosurg 71:ons133–ons145
Keles G, Lamborn K, Berger M (2001) Low-grade hemispheric gliomas in adults: a critical review of extent of resection as a factor influencing outcome. J Neurosurg 95(5):735–745
Kurimoto M, Hayashi N, Kamiyama H, Nagai S, Shibata T, Asahi T, Matsumura N, Hirashima Y, Endo S (2004) Impact of neuronavigation and image-guided extensive resection for adult patients with supratentorial malignant astrocytomas: a single-institution retrospective study. Minim Invasive Neurosurg 47(5):278–283
Lacroix M, Abi-Said D, Fourney D et al (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95(2):190–198
Lacroix M, Toms S (2014) Maximum safe resection of glioblastoma multiforme. J Clin Oncol 32(8):727–728. https://doi.org/10.1200/JCO.2013.53.2788
McGirt M, Chaichana K, Gathinji M, Attenello F, Than K, Olivi A, Weingart J, Brem H, Quiñones-Hinojosa A (2009) Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg 110(1):156–162. https://doi.org/10.3171/2008.4.17536
Missios S, Bekelis K, Barnett GH (2015) Renaissance of laser interstitial thermal ablation. Neurosurg Focus 38(3):E13. https://doi.org/10.3171/2014.12.FOCUS14762
Norred S, Johnson J (2014) Magnetic resonance-guided laser induced thermal therapy for glioblastoma multiforme: a review. Biomed Res Int 2014:1–9. https://doi.org/10.1155/2014/761312
Pisipati S, Smith K, Shah K, Ebersole K, Chamoun R, Camarata P (2016) Intracerebral laser interstitial thermal therapy followed by tumor resection to minimize cerebral edema. Neurosurg Focus 41(4):E13. https://doi.org/10.3171/2016.7.FOCUS16224
Rahmathulla G, Recinos PF, Kamian K, Mohammadi AM, Ahluwalia MS, Barnett GH (2014) MRI-guided laser interstitial thermal therapy in neuro-oncology: a review of its current clinical applications. Oncology 87(2):67–82. https://doi.org/10.1159/000362817
Rosomoff H, Carroll F (1966) Reaction of neoplasm and brain to laser. Arch Neurol 14(2):143–148. https://doi.org/10.1001/archneur.1966.00470080027004
Roux F, Devaux B, Merienne L, Cioloca C, Chodkiewicz J (1990) 1.32 μm Nd:YAG laser during neurosurgical procedures experience with about 70 patients operated on with the MC 2100 unit. Acta Neurochir 107(3–4):161–166
Roux F, Mordon S, Fallet-Bianco C, Merienne L, Devaux B, Chodkiewicz J (1990) Effects of 1.32-μm Nd-YAG laser on brain thermal and histological experimental data. Surg Neurol 34(6):402–407. https://doi.org/10.1016/0090-3019(90)90244-J
Sawaya R, Hammoud M, Schoppa D, Hess K, Wu S, Shi W, WiIdrick D (1998) Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery 42(5):1044–1055. https://doi.org/10.1097/00006123-199805000-00054
Schober R, Bettag M, Sabel M, Ulrich F, Hessel S (1993) Fine structure of zonal changes in experimental Nd:YAG laser–induced interstitial hyperthermia. Lasers Surg Med 13(2):234–241
Schulze P et al (2001) Correlation of neuropathologic findings and phase-based MRI temperature maps in experimental laser-induced interstitial thermotherapy. J Magn Reson Imaging 14(5):658–658. https://doi.org/10.1002/jmri.1232
Senft C, Forster M, Bink A, Mittelbronn M, Franz K, Seifert V, Szelényi A (2012) Optimizing the extent of resection in eloquently located gliomas by combining intraoperative MRI guidance with intraoperative neurophysiological monitoring. J Neuro-Oncol 109(1):81–90
Sloan A, Ahluwalia M, Valerio-Pascua J et al (2013) Results of the NeuroBlate System first-in-humans Phase I clinical trial for recurrent glioblastoma. J Neurosurg 118(6):1202–1219
Stellar S, Polanyi T, Bredemeier H (1970) Experimental studies with the carbon dioxide laser as a neurosurgical instrument. Med Biol Eng 8(6):549–558. https://doi.org/10.1007/BF02478229
Stummer W, Reulen H, Meinel T et al (2008) Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery 62(3):564–576
Svaasand L, Boerslid T, Oeveraasen M (1985) Thermal and optical properties of living tissue: application to laser-induced hyperthermia. Lasers Surg Med 5(6):589–602
Takizawa T (1984) The carbon dioxide laser surgical unit as an instrument for surgery of brain tumours—its advantages and disadvantages. Neurosurg Rev 7(2–3):135–144. https://doi.org/10.1007/BF01780696
Wolbers J (2014) Novel strategies in glioblastoma surgery aim at safe, supra-maximum resection in conjunction with local therapies. Chin J Cancer 33(1):8–15. https://doi.org/10.5732/cjc.013.10219
Yaroslavsky AN, Schulze PC, Yaroslavsky IV, Schober R, Ulrich F, Schwarzmaier HJ (2002) Optical properties of selected native and coagulated human brain tissues in vitro in the visible and near infrared spectral range. Phys Med Biol 47(12):2059–2073. https://doi.org/10.1088/0031-9155/47/12/305
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved and conducted within the guidelines of the institutional review board.
Informed consent
The written informed consent was obtained from each patient or appropriate family member before the surgery.
Rights and permissions
About this article
Cite this article
Rozumenko, A., Kliuchka, V., Rozumenko, V. et al. Image-guided resection of glioblastoma in eloquent brain areas facilitated by laser surface thermal therapy: clinical outcomes and long-term results. Neurosurg Rev 41, 1045–1052 (2018). https://doi.org/10.1007/s10143-018-0948-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-018-0948-y