Abstract
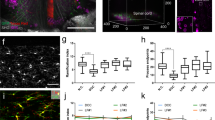
The development of imaging technique to visualize and quantify the structural alteration of the spinal cord injury (SCI) may lead to better understanding and treatments of the injuries. In this work, multiphoton microscopy (MPM) based on two-photon excited fluorescence (TPEF) and second-harmonic generation (SHG) was tentatively applied to quantitatively visualize the cellular microstructures of SCI to demonstrate the feasibility and superiority of MPM in SCI imaging. High-contrast MPM images of normal and injured spinal cord tissue were obtained for comparison. Moreover, the changes of injured spinal cord were characterized by the quantitative analysis of the MPM images. These results showed that MPM combined with quantitative method has the ability to identify the characteristics of spinal cord injury including the changes in the contents of nerve fibers and extracellular matrix. With the advancement of MPM, we believe that this technique has great potential to provide the histological diagnosis for the monitoring and evaluation of SCI.







Similar content being viewed by others
References
Galli R, Uckermann O, Winterhalder MJ, Sitoci-Ficici KH, Geiger KD, Koch E, Schackert G, Zumbusch A, Steiner G, Kirsch M (2012) Vibrational spectroscopic imaging and multiphoton microscopy of spinal cord injury. Anal Chem 84:8707–8714
Galtrey CM, Kwok JC, Carulli D, Rhodes KE, Fawcett JW (2008) Distribution and synthesis of extracellular matrix proteoglycans, hyaluronan, link proteins and tenascin-R in the rat spinal cord. Eur J Neurosci 27:1373–1390
Wiese S, Faissner A (2015) The role of extracellular matrix in spinal cord development. Exp Neurol 274:90–99
Gaudet AD, Popovich PG (2014) Extracellular matrix regulation of inflammation in the healthy and injured spinal cord. Exp Neurol 258:24–34
Ellingson BM, Salamon N, Holly LT (2014) Imaging techniques in spinal cord injury. World Neurosurg 82:1351–1358
Powers BE, Sellers DL, Lovelett EA, Cheung W, Aalami SP, Zapertov N, Maris DO, Horner PJ (2013) Remyelination reporter reveals prolonged refinement of spontaneously regenerated myelin. Proc Natl Acad Sci U S A 110:4075–4080
Petersen JA, Wilm BJ, von Meyenburg J, Schubert M, Seifert B, Najafi Y, Dietz V, Kollias S (2012) Chronic cervical spinal cord injury: DTI correlates with clinical and electrophysiological measures. J Neurotrauma 29:1556–1566
Wang M, Dai Y, Han Y, Haacke EM, Dai J, Shi D (2011) Susceptibility weighted imaging in detecting hemorrhage in acute cervical spinal cord injury. Magn Reson Imaging 29:365–373
Kang CE, Clarkson R, Tator CH, Yeung IW, Shoichet MS (2010) Spinal cord blood flow and blood vessel permeability measured by dynamic computed tomography imaging in rats after localized delivery of fibroblast growth factor. J Neurotrauma 27:2041–2053
Zoumi A, Yeh A, Tromberg BJ (2002) Imaging cells and extracellular matrix in vivo by using second-harmonic generation and two photon excited fluorescence. Proc Natl Acad Sci U S A 99:11014e9
Uckermann O, Galli R, Beiermeister R, Sitoci-Ficici KH, Later R, Leipnitz E, Neuwirth A, Chavakis T, Koch E, Schackert G, Steiner G, Kirsch M (2015) Endogenous two-photon excited fluorescence provides label-free visualization of the inflammatory response in the rodent spinal cord. Biomed Res Int 2015:859084
Israel JS, Esquibel CR, Dingle AM, Liu Y, Keikhosravi A, Pisaniello JA, Hesse MA, Brodnick SK, Novello J, Krugner-Higby L, Williams JC, Eliceiri KW, Poore SO (2017) Quantification of collagen organization after nerve repair. Plast Reconstr Surg Glob Open 5:e1586
Noble LJ, Wrathall JR (1985) Spinal cord contusion in the rat: morphometric analyses of alterations in the spinal cord. Exp Neurol 88:135–149
Zhu X, Tang Y, Chen J, Xiong S, Zhuo S (2013) Monitoring wound healing of elastic cartilage using multiphoton microscopy. Osteoarthr Cartilage 21:1799–1806
Quinn KP, Georgakoudi I (2013) Rapid quantification of pixel-wise fiber orientation data in micrographs. J Biomed Opt 18:046003–046003
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biometrika 52:591–611
Nam MH, Baek M, Lim J, Lee S, Yoon J, Kim J, Soh KS (2014) Discovery of a novel fibrous tissue in the spinal pia mater by polarized light microscopy. Connect Tissue Res 55:147–155
Bignami A, Asher R, Perides G (1992) The extracellular matrix of rat spinal cord: a comparative study on the localization of hyaluronic acid, glial hyaluronate-binding protein, and chondroitin sulfate proteoglycan. Exp Neurol 117:90–93
Li D, Zheng W, Zeng Y, Luo Y, Qu JY (2011) Two-photon excited hemoglobin fluorescence provides contrast mechanism for label-free imaging of microvasculature in vivo. Opt Lett 36:834–836
Saytashev I, Glenn R, Murashova GA, Osseiran S, Spence D, Evans CL, Dantus M (2016) Multiphoton excited hemoglobin fluorescence and third harmonic generation for non-invasive microscopy of stored blood. Biomed Opt Express 7:3449–3460
Liao CX, Wang ZY, Zhou Y, Zhou LQ, Zhu XQ, Liu WG, Chen JX (2017) Label-free identification of the microstructure of rat spinal cords based on nonlinear optical microscopy. J Microsc 267:143–149
Waxman SG, Kocsis JD, Stys PK (1995) The axon: structure, function, and pathophysiology. Oxford University Press, Oxford
Wang F, Bélanger E, Paquet ME, Côté DC, De Koninck Y (2016) Probing pain pathways with light. Neuroscience 338:248–271
Johansen-Berg H, Behrens TE (2013) Diffusion MRI: from quantitative measurement to in vivo neuroanatomy. Academic press, San Diego
Cheran S, Shanmuganathan K, Zhuo J, Mirvis SE, Aarabi B, Alexander MT, Gullapalli RP (2011) Correlation of MR diffusion tensor imaging parameters with ASIA motor scores in hemorrhagic and nonhemorrhagic acute spinal cord injury. J Neurotrauma 28:1881–1892
Quencer RM, Bunge RP, Egnor M, Green BA, Puckett W, Naidich TP, Post MJD, Norenberg M (1992) Acute traumatic central cord syndrome: MRI-pathological correlations. Neuroradiology 34:85–94
Douek P, Turner R, Pekar J, Patronas N, Le Bihan D (1991) MR color mapping of myelin fiber orientation. J Comput Assist Tomogr 15:923–929
Li LM, Li JB, Zhu Y, Fan GY (2010) Soluble complement receptor type 1 inhibits complement system activation and improves motor function in acute spinal cord injury. Spinal Cord 48:105–111
Dray C, Rougon G, Debarbieux F (2009) Quantitative analysis by in vivo imaging of the dynamics of vascular and axonal networks in injured mouse spinal cord. Proc Natl Acad Sci U S A 106:9459–9464
Helmchen F, Denk W, Kerr JN (2013) Miniaturization of two-photon microscopy for imaging in freely moving animals. Cold Spring Harb Protoc 2013:904–913
Funding
The work was supported by the Changjiang Scholars Program and the University Innovative Research Team (grant no. IRT_15R10), the National Natural Science Foundation of China (grant nos. 81671730), the Natural Science Foundation of Fujian Province (2015J01241), and a grant from the Education Bureau of Fujian Province (grant no. JA13060).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The experimental procedures with rats were conducted according to the Guide for Care and Use of Laboratory Animals and approved by the Animal Care and Use Committee in Fujian Medical University.
Informed consent
It does not apply, since the study was developed with Sprague-Dawley rats.
Rights and permissions
About this article
Cite this article
Liao, C., Zhu, X., Zhou, L. et al. Visualize and quantify the structural alteration of the rat spinal cord injury based on multiphoton microscopy. Lasers Med Sci 34, 561–569 (2019). https://doi.org/10.1007/s10103-018-2630-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-018-2630-6