Abstract
The aim of the study was to assess reinfection rates in relation to long-term antibody dynamics against SARS-CoV-2 after the first wave. A prospective longitudinal study with monthly serological follow-up during the first 4 months, and then at 6, 8, and 10 months after the disease onset of all recovered adult in- and outpatients with COVID-19 attending Udine Hospital (Italy) from March to May 2020. During the follow-up, reinfections were collected. A total of 546 unselected individuals with COVID-19 acquired from March to May 2020 were included (292 female, mean age 53 years). After a median follow-up of 10 months (IQR 6.2–10.4), reinfection occurred in 6 (1.1%) patients, median age of 44.5 years (IQR 33‒49). All had a previous history of mild COVID-19 (all were healthcare workers) and reinfection occurred a median of 9 months (IQR 8.2‒10.2) after the onset of the first episode. Patients with reinfection were either seronegative (2/56, n = 3.6%), seroreverted (2/137, 1.5%), or seropositive (2/353, 0.6%) (p = 0.085). All reinfections were mild (n = 5) or asymptomatic (n = 1). After reinfection, none of patients developed IgM response and only two had a transitory boosted IgG immunization response. In an unselected population after the first wave of COVID-19, after a prolonged observation period (mean 10 months), reinfection was very uncommon; occurred in patients with a previous history of mild infection, mostly with weak or absent serological response; and manifested with mild or asymptomatic clinical presentation.
Similar content being viewed by others
Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) humoral immunity suggests that more than 90% of seroconversion rates occur after acute primary infection with variable degrees of decline in antibody levels over time [14,15,1,2,3,4,5,6,7,16].
The aim of this prospective longitudinal study was to comprehensively characterize the relative incidence of COVID-19 reinfections in relation to serological response among individuals who had recovered from COVID-19 after the first wave. The study included a wide spectrum of unselected patients ranging from asymptomatic to severely infected, assessed over a 10-month follow-up period.
Methods
Study setting and patient population
,17].
The target population was a cohort of all consecutive adult in- and outpatients (≥ 18 years) attending the Infectious Disease Department with a diagnosis of COVID-19 from March 1 (the day of the first COVID-19 diagnosis at our hospital) until May 30, 2020. Further definitions of acute COVID-19 and baseline conditions are summarized in Supplementary Table 1.
Serological test collection, reinfection follow-up, and definitions
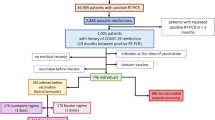
SARS-CoV-2 antibody concentrations were measured at the serological follow-up visits each month (± 15 days) after symptom onset for the first 4 months, and every other month up to 10 months (± 15 days), from March 2020 to February 2021 (CORMOR 3–4® protocol). Patients attending at least two serological follow-ups were included in the study (Fig. 1).
,18].
Specifically, patients with symptoms or signs of recurrent illness (fever, rhinorrhea, sore throat, cough, dyspnea, sputum, myalgia, fatigue, thoracic pain, vomiting, diarrhea, dysgeusia or anosmia, conjunctivitis, rash) and/or a positive PCR test for SARS-CoV-2 were instructed to contact the research team by phone, in order to schedule a prompt visit (within 24 h) at the infectious disease outpatient clinic or go to the emergency department for medical examination and PCR test for SARS-CoV-2. In addition, during this contact, patients were asked about previous not-reported episodes of symptoms/signs. Systematic SARS-CoV-2 PCR test was performed at regular intervals (every 2/4 weeks) only for healthcare workers (HCWs) in accordance to Hospital and Nursing homes/long-term facility protocols.
At the time of reinfection, for definitive analysis, we classified patients according to their most recent (within 2 months) antibody status into three groups: (1) seronegative in the absence of any IgM-/IgG-positive serological samples; (2) seroreverted in the presence of a decline in IgM/IgG antibody levels below the positivity threshold after initial seroconversion; and (3) seropositive in the presence of persistence of IgM-/IgG-positive serological sample.
Antibody measurements
Serum concentrations of the anti-SARS-CoV-2 specific antibodies IgG and IgM were assessed using iFlash-SARS-CoV-2 (Shenzhen YHLO Biotech Co., Ltd., China, distributed in Italy by Pantec SRL), a paramagnetic particle chemiluminescence immunoassay (CLIA) for the determination of IgM and IgG antibodies against SARS-CoV-2 N and S protein. In accordance with the manufacturer’s instructions, the IgM and IgG thresholds for positivity were considered to be 10.0 kAU/L.19.
Statistical analysis
Patients’ demographic and clinical characteristics were presented with absolute values and percentages for categorical variables and means or medians (standard deviation (SD) or interquartile ranges (IQRs)) for continuous variables. The Shapiro–Wilk test was used to assess whether data were normally or non-normally distributed. Categorical variables were compared using the chi square (χ2) test or Fisher’s exact test, while quantitative variables were compared using the t-test or Mann–Whitney U test, as appropriate. Statistical analysis was performed using STATA 16.1.
Results
Study population at onset of acute COVID-19
Overall, during the study period, a total of 1067 patients received the COVID-19 diagnosis. After excluding 211 patients for refusing to participate in the research, 138 nursing home/long-term facility residents who were not capable of giving their consent due to cognitive decline, 38 who were lost to follow-up, 51 for incomplete serological follow-up and 81 deaths, a total of 546 patients were included (Fig. 1). Demographic and clinical characteristics of patients at baseline are summarized in Table 1. The mean age of our study population was 53 years (SD 15.4; range 18‒94), 292 (53.5%) were female and the majority (480/521, 92.1%) were native Italians. One hundred and fifteen were HCWs. During the acute phase, most patients (502, 91.9%) were symptomatic and presented mild illness (374, 68.5%). One hundred and forty-seven (27.2%) had been hospitalized (22 in the intensive care unit) (Table 1).
Serological dynamics of SARS-CoV-2 IgM and IgG after primary infection
A complete description of the serological evolution of the study population is presented in another work that is currently in progress. In brief, the overall seroconversion rate within 2 months was 32% for IgM: 25% in mild cases and 61% in moderate to critical cases. IgM was generally not detected after 4 months (90th percentile equal to 135 days). The overall seroconversion rate for IgG within 2 months was higher for IgG (90%): 91% in mild patients, 100% in moderate to critical patients, and only 54% in asymptomatic cases. About half of the patients (47%) had experienced IgG seroreversion at 10 months, and rates of antibody loss were almost complete (88%) for asymptomatic patients, around half for mild cases (53%) but only 13% for moderate to severe COVID-19.
Reinfection
Patients were followed up for a median of 10 months (IQR, 6.2–10.4). The reinfection rate was 1.1% (6/546). Cases of reinfection occurred at a median of 9 months (IQR 8.2‒10.2) after the acute onset of the first episode. The median age was 44.5 years (IQR 33‒49) and all were HCWs (Table 2). As reported in Table 2, all patients experienced mild infection (6/370, 1.6%) during the first episode and manifested mild (n = 5) or asymptomatic (n = 1) reinfections. Reinfection rates did not differ significantly in seronegative (2/56, n = 3.6%), seroreverted (2/137, 1.5%), or seropositive (2/353, 0.6%) patients (p = 0.085) but were significantly higher in HCWs than in non-HCWs (6/119, 5.0% versus 0/385, p < 0.001). Only one patient had a high-titer serological response against SARS-CoV-2 at the time of reinfection (Table 2) (Fig. 2). After reinfection, none of the patients developed an IgM response and only two had a transitory boosted IgG immunization response (Fig. 2). After repeating isolation and tracing of close contacts, we found no transmission to other individuals. The serological evolution after the first and second infections is described in Fig. 2.
Discussion
Our prospective longitudinal study of an unselected population with COVID-19, acquired during the first wave of the pandemic, with different degrees of severity, shows that there was a very low risk of reinfection after a mean follow-up of 10 months and that the primary serological response was not accurately predictive of reinfection.
The fact that all our reinfections occurred in HCWs is surprising. This could be due either to a higher grade of exposure to COVID-19 cases or to a more rapid access to SARS-CoV-2 molecular PCR testing compared with the general population [1,2,6,12,18].
It is worth noting that although there is currently no strong evidence that previous SARS-CoV-2 infection reduces the transmission risk [23,14,23,1,7,10,18,25,1,2,3,4,5,6,7,8,9,10,14,24,27,28
,].
,7,
Conclusions
Most people who have recovered from COVID-19 have a low risk of reinfection; secondary infections may occur mainly in patients with a primary mild COVID-19 infection and with low or absent serological response at the time of reinfection. These findings may suggest that natural humoral protective immunity may be transient and may not confer herd immunity. Given the short supply of vaccine in some countries or settings, public health interventions should be extended as not to prioritize patients with a previous history of SARS-CoV-2 infection except for those at risk for poor outcomes and/or high-risk settings of exposure. Further large-scale standardized longitudinal studies with a longer follow-up focused both on humoral and on cell-mediated adaptive SARS-CoV-2 immunity are needed to determine the longevity of protection after infection for subsequent episodes in order to understand the evolution of the pandemic and to design a vaccination plan, taking into consideration emerging variants of concern.
Supplementary information
The online version contains the supplementary material.
Data availability
Original data will be made available upon request.
Code availability
Not applicable.
Abbreviations
- BMI:
-
Body mass index
- COVID-19:
-
Coronavirus Disease 2019
- ICU:
-
Intensive care unit
- IQR:
-
Interquartile range
- HCW:
-
Healthcare worker
- SARS-CoV-2:
-
Severe Acute Respiratory Syndrome Coronavirus 2
- SD:
-
Standard deviation
References
Babiker A, Marvil CE, Waggoner JJ, Collins MH, Piantadosi A. The importance and challenges of identifying SARS-CoV-2 reinfections. Journal of clinical microbiology. 2021;59(4).
Lumley SF, O’Donnell D, Stoesser NE et al (2021) Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med 384(6):533–540
Hall VJ, Foulkes S, Charlett A et al (2021) SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN). Lancet 397(10283):1459–1469
Addetia A, Crawford KHD, Dingens A, et al. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. Journal of clinical microbiology. ct 21 2020;58(11).
Cavanaugh AM, Thoroughman D, Miranda H, Spicer K (2021) Suspected recurrent SARS-CoV-2 infections among residents of a skilled nursing facility during a second COVID-19 outbreak - Kentucky, July-November 2020. MMWR Morb Mortal Wkly Rep 70(8):273–277
Abu-Raddad LJ, Chemaitelly H, Coyle P, et al. SARS-CoV-2 reinfection in a cohort of 43,000 antibody-positive individuals followed for up to 35 weeks. medRxiv : the preprint server for health sciences. 2021:2021.2001.2015.21249731.
Harvey RA, Rassen JA, Kabelac CA et al (2021) Association of SARS-CoV-2 seropositive antibody test with risk of future infection. JAMA Intern Med 181(5):672–679
Hansen CH, Michlmayr D, Gubbels SM, Molbak K, Ethelberg S (2021) Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet 397(10280):1204–1212
Hanrath AT, Payne BAI, Duncan CJA (Apr 2021) Prior SARS-CoV-2 infection is associated with protection against symptomatic reinfection. J Infect 82(4):e29–e30
Letizia AG, Ge Y, Vangeti S, et al. SARS-CoV-2 seropositivity and subsequent infection risk in healthy young adults: a prospective cohort study. The Lancet. Respiratory medicine. 2021.
Graham MS, Sudre CH, May A, et al. Changes in symptomatology, reinfection, and transmissibility associated with the SARS-CoV-2 variant B.1.1.7: an ecological study. The Lancet. Public health. 2021;6(5):e335-e345.
Adrielle Dos Santos L, Filho PGG, Silva AMF, et al. Recurrent COVID-19 including evidence of reinfection and enhanced severity in thirty Brazilian healthcare workers. The Journal of infection. 2021;82(3):399–406.
Rennert L, McMahan C. Risk of SARS-CoV-2 reinfection in a university student population. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. May 16 2021.
Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529).
Ibarrondo FJ, Fulcher JA, Goodman-Meza D et al (2020) Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild Covid-19. N Engl J Med 383(11):1085–1087
Anichini G, Terrosi C, Gandolfo C, et al. SARS-CoV-2 antibody response in persons with past natural infection. The New England journal of medicine. Apr 14 2021.
Vandenbroucke JP, von Elm E, Altman DG et al (Dec 2014) Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg 12(12):1500–1524
Yahav D, Yelin D, Eckerle I et al (Mar 2021) Definitions for coronavirus disease 2019 reinfection, relapse and PCR re-positivity. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases 27(3):315–318
Plebani M, Padoan A, Negrini D, Carpinteri B, Sciacovelli L. Diagnostic performances and thresholds: the key to harmonization in serological SARS-CoV-2 assays? Clinica chimica acta; international journal of clinical chemistry. Oct 2020;509:1–7.
Centers for Disease Control and Prevention Reinfection with COVID-19. 2020 Oct 27. [cited 2021 April 27]. https://www.cdc.gov/coronavirus/2019-ncov/your-health/reinfection.html
https://www.hiqa.ie/sites/default/files/2021-03/Duration-of-protective-immunity_Evidence-Summary.pdf HIaQAMcA.
Resende PC, Bezerra JF, Teixeira Vasconcelos RH, et al. Severe Acute Respiratory Syndrome Coronavirus 2 P.2 lineage associated with reinfection case, Brazil, June-October 2020. Emerging infectious diseases. 2021;27(7).
Jeffery-Smith A, Iyanger N, Williams SV, et al. Antibodies to SARS-CoV-2 protect against re-infection during outbreaks in care homes, September and October 2020. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2021;26(5).
Britton A, Jacobs Slifka KM, Edens C et al (2021) Effectiveness of the Pfizer-BioNTech COVID-19 vaccine among residents of two skilled nursing facilities experiencing COVID-19 outbreaks - Connecticut, December 2020-February 2021. MMWR Morb Mortal Wkly Rep 70(11):396–401
Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593(7857):130–135.
To KK, Hung IF, Chan KH, et al. Serum antibody profile of a patient with Coronavirus Disease 2019 reinfection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2021;72(10):e659-e662.77777
Acknowledgements
The authors would like to thank all clinical and nursing staff who cared for the patients at Udine Infectious Disease Clinic during hospitalization and ambulatory management. We also thank Rodolfo Sbrojavacca for his precious and strenuous support in the fight against COVID-19.
Funding
This research was funded by PRIN 2017 n.20178S4EK9—“Innovative statistical methods in biomedical research on biomarkers: from their identification to their use in clinical practice.” Maria de Martino research was supported by the grant.
Author information
Authors and Affiliations
Contributions
Maddalena Peghin: conceptualization, methodology, formal analysis, investigation, and writing—review and editing. Emilio Bouza: conceptualization and writing—review; Martina Fabris: laboratory analysis and review; Maria De Martino: conceptualization, methodology, formal analysis, and writing; Alvisa Palese: conceptualization and writing—review and editing; Giulia Bontempo: data collections; Elena Graziano: data collections; Valentina Gerussi: data collections; Valentina Bressan: data collections; Assunta Sartor: laboratory analysis; Miriam Isola: conceptualization, methodology, formal analysis, and writing; Carlo Tascini: methodology, formal analysis, investigation, writing—review and editing; and Francesco Curcio: laboratory analysis and review.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Ethics Committee of the Friuli Venezia Giulia Region (CORMOR 3–4 protocol; CEUR-2020-OS-219; and CEUR-2020-OS-205). All procedures were carried out in accordance with the ethical standards of the University of Udine and the ASUFC and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all subjects before collecting the data and performing serological tests.
Consent to participate
Oral and written informed consent was obtained from the participants.
Consent for publication
The authors have seen the final version of the manuscript and approved submission for publication.
Conflict of interest
MP reports receiving grants and personal fees from Pfizer, MSD, Thermofisher and Dia Sorin outside the submitted work. CT has received grants in the last two years from Correvio, Biotest, Biomerieux, Gilead, Angelini, MSD, Pfizer, Thermofisher, Zambon, Shionogi, Avir Pharma, and Hikma unrelated to the study submitted. The other authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peghin, M., Bouza, E., Fabris, M. et al. Low risk of reinfections and relation with serological response after recovery from the first wave of COVID-19. Eur J Clin Microbiol Infect Dis 40, 2597–2604 (2021). https://doi.org/10.1007/s10096-021-04335-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-021-04335-x