Abstract
Influenza was recently reported as a risk factor for invasive aspergillosis (IA). We aimed to describe prognostic factors for influenza-associated IA (IAA) and poor outcome and mortality in critically ill patients in Switzerland. All adults with confirmed influenza admitted to the ICU at two Swiss tertiary care centres during the 2017/2018 influenza season were retrospectively evaluated. IAA was defined by clinical, mycological and radiological criteria: a positive galactomannan in bronchoalveolar lavage or histopathological or cultural evidence in respiratory specimens of Aspergillus spp., any radiological infiltrate and a compatible clinical presentation. Poor outcome was defined as a composite of in-hospital mortality, ICU length of stay (LOS), invasive ventilation for > 7 days or extracorporeal membrane oxygenation. Of 81 patients with influenza in the ICU, 9 (11%) were diagnosed with IAA. All patients with IAA had poor outcome compared to 26 (36%) patients without IAA (p < 0.001). Median ICU-LOS and mortality were 17 vs. 3 days (p < 0.01) and 3/9 (33%) vs. 13/72 (18%; p = 0.37) in patients with vs. without IAA, respectively. Patients with IAA had significantly longer durations of antibiotic therapy, vasoactive support and mechanical ventilation. Aspergillus was the most common respiratory co-pathogen (9/40, 22%) followed by classical bacterial co-pathogens. IAA was not associated with classical risk factors. Aspergillus is a common superinfection in critically ill influenza patients associated with poor outcome and longer duration of organ supportive therapies. Given the absence of classical risk factors for aspergillosis, greater awareness is necessary, particularly in those requiring organ supportive therapies.
Similar content being viewed by others
Introduction
Influenza infection has been recently defined as a risk factor of invasive aspergillosis [12, 26]. Influenza can cause severe pneumonia and acute respiratory distress syndrome (ARDS) [17, 31, 35]. Respiratory bacterial superinfection of influenza represents a common complication with high mortality [15, 18, 25, 29, 30]. Bacterial pathogens superinfecting influenza pneumonia have changed over time probably as a function of both different hosts and influenza strains. These include Haemophilus influenzae, Streptococcus pyogenes, Staphylococcus aureus and Streptococcus pneumoniae [18]. S. pneumoniae was the most common pathogen in the 1918 pandemic [29] and S. aureus emerged as another frequent co-pathogen during the 2009/2010 H1N1 pandemic [25, 30]. High prevalence of Pseudomonas aeruginosa superinfection has been reported in intensive care unit (ICU) patients [15, 16]. Superinfection with Aspergillus spp. has been increasingly described since the 2009/2010 influenza pandemic [2, 10, 13, 36] associated with even higher mortality (33–67%) [7, 11, 26, 32, 36]. IAA is independent of influenza type (A or B [20, 26]) and also affects immunocompetent hosts. Between 10–23% of influenza patients in the ICU [26, 32, 36], less frequently in North America (7.2%) [27] and 32% of immunocompromised influenza patients have been reported to have IAA [26]. Predictors of IAA, poor outcome and mortality in influenza patients in the ICU remain unknown. Since no data on IAA was available from Switzerland, we retrospectively analysed all patients with severe influenza infection needing treatment in two large Swiss ICUs during the 2017/2018 influenza season with regard to predictors of IAA, mortality and poor outcome.
Methods
Study design, participants and outcomes
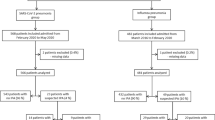
In this retrospective cohort study, sixteen ICUs of tertiary hospitals in Switzerland were asked if they had observed cases of IAA and severe influenza and routinely looked for IAA based on clinical suspicion with galactomannan and fungal cultures in BAL; only two of them met the criteria (Cantonal Hospital of St. Gallen and University Hospital of Geneva). All adults (≥ 18 years) with confirmed influenza infection during the 2017/2018 influenza season (December 2017–April 2018) admitted to the ICU for ≥ 24 h of those centres were included. Patients whose influenza diagnosis occurred after the discharge from the ICU were excluded. Influenza infection was diagnosed by polymerase chain reaction (PCR) from nasopharyngeal swab, sputum or bronchoalveolar lavage (BAL). Patients were identified from the ICU databases, infectious diseases and hospital epidemiology databases in order to improve identification of patients and reduce reporting bias. The study was approved by the local ethics committees (EKOS 2018-01994). There was no funding.
Endpoints
The primary aim was to identify predictors of IAA in critically ill patients with influenza infection. Secondary aims were to detect predictors of mortality and poor outcome of severe influenza infection. Poor outcome was defined as a composite of ICU length of stay (LOS) ≥ 7 days, need of extracorporeal membrane oxygenation (ECMO), invasive ventilation for ≥ 7 days or in-hospital death.
ARDS was diagnosed according to the Berlin criteria [3, 4].
Outcome definitions
-
A.
Definitions
IAA was defined by clinical, radiological and mycological criteria according to the modified criteria of IAA by Schauwvlieghe et al. and Blot et al. (Supplementary table 1) [5, 26]. Records of all IAA patients were reviewed and consensus was achieved by investigators from both centres whether criteria of IAA were fulfilled. The Platelia assay “Aspergillus Ag” (Biorad) was used to detect GM.
Respiratory superinfection was defined as (I) detection of clinically relevant bacterial or fungal pathogen in respiratory specimens or positive blood cultures that was treated with antibiotics or antifungals, respectively, (II) a positive pneumococcal urinary antigen, Pneumocystis jirovecii antigen in BAL or positive GM in serum or BAL (Table 1 in the Appendix). Other superinfection included all respiratory superinfections, bacteraemia, catheter-associated infections and Clostridioides difficile colitis.
Organ supportive therapies were defined as need of renal replacement therapy, vasoactive support, invasive mechanical ventilation and ECMO.
Statistical analysis
Continuous variables were assessed by t tests or Mann-Whitney U tests as appropriate. For categorical values, comparison was done by Fisher’s exact test. Missing data were not imputed. A two-sided p value < 0.05 was considered as statistically significant. Given the small number of each outcome, only the univariate but not the multivariable analyses are reported. Data analysis was performed with SAS (version 9.3, SAS Institute Inc., Cary, NC, USA) and R core team (2017, R foundation for statistical computing, Vienna, Austria).
Results
Patient characteristics
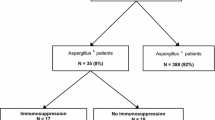
Of 91 patients identified from the databases, 10 were excluded (5 patients per centre) and 81 were included and analysed. Median follow-up time was 17 days. Nine patients had IAA (11%). There were no statistically significant differences in baseline characteristics between patients with and without IAA (Table 1 in the Appendix). Immunosuppressive diseases were uncommon in both groups. During hospitalization, corticosteroids were initiated in half of all influenza patients (78% vs. 46% in IAA and non-IAA, p = 0.14). 5/37 (13.5%) patients with influenza A had IAA and 4/44 (9%) with influenza B (p = 0.55).
Duration of influenza symptoms before diagnosis of influenza was significantly longer in those with IAA (median duration 9 days (interquartile range (IQR) 5–14) vs. 5 days (IQR 4–8) in non-IAA, p = 0.03), whereas there was no difference in duration of symptoms until hospitalization (median duration 8 days (IQR 4–11) in IAA and 4 days (IQR 3–8) in non-IAA, p = 0.11). IAA symptoms started after a median of 3 days after ICU admission. IAA was diagnosed after a median of 7 days after ICU admission and 11 days after first symptoms of influenza (IQR 3–8 and 10–18 days respectively). GM was measured in 33% of all patients (89% in IAA and 26% in non-IAA, p < 0.001). GM was positive in 1 of 19 (5.3%) patients in non-IAA and 7 of 8 (88%) samples with IAA (p < 0.001). All patients with positive GM had antibiotics prior to GM testing, as had 14 of 16 (87.5%) patients with a negative GM (p = 0.52). Two patients with IAA and negative GM had therapy with voriconazole started on the same day as GM testing was performed. It cannot be excluded that treatment was started before BAL was performed which could have caused in a false-negative test result. Eight of 9 patients with IAA had radiologic infiltrates and 4 nodules. There were no positive cultures of Aspergillus spp. in non-IAA patients but growth of Aspergillus spp. in 8 (89%) patients with IAA, mainly Aspergillus fumigatus (7 of 8 patients).
Severity and outcome of illness
Organ supportive therapies and complications were more frequent in IAA patients than in non-IAA patients (Table 2 in the Appendix). Median duration of antibiotic treatment (16 vs. 6.5 days), invasive mechanical ventilation (13 vs. 0 days), vasoactive support (7 vs. 0 days), hospital-LOS (40 vs. 15 days) and ICU-LOS (17 vs. 3 days) were significantly extended in IAA compared with non-IAA patients (Fig. 1). The length of stay in the ICU was significantly elevated in IAA patients (Fig. 2). Treatment with antivirals (oseltamivir with or without zanamivir) was initiated in all patients with IAA and 93% of non-IAA patients (p = 1.0). Mold-active antifungal treatment was initiated in 16 patients (8 patients each with IAA and non-IAA because of presumed IAA during hospitalisation). Median duration of antifungal treatment was 10 days (IQR 1.5–25.5) in IAA. Antifungal treatment was stopped when diagnostics were negative in those without IAA. Corticosteroids were used during hospitalisation in 60.5% of patients (89% in IAA vs. 20.5% in non-IAA, p = 0.12) and before influenza diagnosis in 3 of 9 of patients with IAA and in 16 of 72 of patients without IAA (33% vs. 22%, p = 0.7). All patients with IAA had poor outcome compared with 36% of non-IAA patients (p < 0.001).
Co-infections
IAA patients had more bacterial or fungal (other than due to Aspergillus spp.) respiratory superinfections (67% vs. 28%, p = 0.002). Other infectious complications were catheter-related bloodstream infections and oropharyngeal and oesophageal candidiasis. Aspergillus (n = 9) was the most frequent respiratory co-pathogen in influenza patients. Other common pathogens were S. aureus, S. pneumoniae, S. pyogenes and Gram-negative bacteria (Fig. 3).
Predictors of mortality and poor outcome
Invasive mechanical ventilation, vasoactive support, ECMO, any complication, respiratory and any superinfection were significantly associated with mortality and poor prognosis (Table 3 in the Appendix).
Discussion
Our study has several main findings. IAA is a severe and relatively frequent complication affecting 11% of patients with influenza treated in two Swiss ICUs. Aspergillus represented the most frequent respiratory co-infection of influenza in this cohort. Patients with influenza were most commonly superinfected with Aspergillus spp. despite lacking classical risk factors of invasive aspergillosis. In our study, all patients with IAA had poor outcome and needed more frequently and longer organ supportive therapies such as invasive mechanical ventilation, renal support and vasopressors. Further risk factors for poor outcome were respiratory superinfections.
IAA patients in our study did not have classical risk factors for aspergillosis such as underlying immunosuppressive disease, hematologic malignancies, solid organ or haematopoietic stem cell transplantation or immunosuppressive medications before hospitalisation. Instead, severe influenza infection was frequently their unique risk factor. The occurrence of IAA independent of classical risk factors of invasive aspergillosis has been previously reported [7, 13, 28, 34]. One study showed higher mortality in immunosuppressed patients with IAA in comparison to immunocompetent patients [26]. Reported risk factors for IAA are male sex and corticosteroid therapy prior to the influenza infection [26, 36]. The latter might explain the relatively high number of patients with obstructive lung diseases [2]. We identified the need for prolonged organ supportive therapies in the ICU as a predictor for IAA. The length of ICU was significantly elevated. Because of small sample size, a competitive risk analysis was not performed. Since IAA can occur in the absence of underlying diseases, requirement of vasoactive therapy, rapidly deteriorating respiratory failure and progressive multiple organ failure might be useful alerts for ICU physicians of IAA in severely ill patients with influenza.
Surprisingly, in our cohort, Aspergillus was identified as the most common respiratory superinfecting pathogen in influenza infection. Aspergillus was seen in 22% of all respiratory co-pathogens in our analysis in contrast to 7.2–8.7% of positive cultures from respiratory specimen reported in the literature [15, 16]. Bacterial pathogens are more frequently reported as influenza co-pathogens than Aspergillus [15, 16]. Bacterial co-infections in influenza showed increasing trends over the last years (from 11.4% in 2009 to 23% in 2015) while Aspergillus isolation from culture was relatively stable at ~ 7% in a study of Spanish ICUs [15]. Bacterial respiratory superinfection was even more common in our analysis (35%). Concurrently, S. aureus and S. pneumoniae were frequently observed in our analysis as were Gram-negative bacteria including P. aeruginosa. Respiratory co-infections were significantly associated with mortality in our univariate analysis. Association of co-infection with S. aureus, P. aeruginosa or Aspergillus spp. with mortality has been described [15]. Therefore, influenza patients at risk for respiratory superinfection need to be identified and early diagnostics and treatment need to be implemented.
The increasing reports of IAA could be due to greater awareness and lower threshold for more and more sensitive diagnostics or indeed an increasing prevalence of this fungal disease. IAA was first reported in 1952 [1] but only received attention of a wider audience in the last decade after the 2009 influenza H1N1 pandemic [2, 10, 13, 16]. An influencing factor could be the wide use of corticosteroids [16] and neuraminidase inhibitors for influenza infections in the ICU. In our analysis, many influenza patients received corticosteroids during hospitalization (60.5%) with a non-significant difference between patients with or without IAA, despite data showing increased mortality of influenza pneumonia with corticosteroid use [21, 37]. Corticosteroid use has been associated with aspergillosis independently of influenza infection [6, 14, 19, 23] and corticosteroid use prior to influenza infection has been associated with IAA [26, 36]. Furthermore, experimental in vitro studies and studies in mice indicate that neuraminidase inhibition decreases immune response by impaired cytokine production in response to Aspergillus spp. This effect is also seen with corticosteroid and neuraminidase treatment [33]. This implies that therapy of influenza with neuraminidase inhibitors and corticosteroids might increase susceptibility and predispose to mold infection in influenza. We hypothesize that a greater use of neuraminidase inhibitors since the 2009 influenza pandemic could have contributed to the emergence of IAA. Alternatively, as recently reported in a retrospective cohort study from Alberta, Canada, from 2014 to 2019, different influenza seasons may be associated with varying rates of IAA. In their study, Schwartz et al. identified a considerably higher rate in the most recent 2018/2019 season [27].
GM values in BAL in our study were slightly lower than in previous reports (67-88% [7, 26, 27] or even 97% among patients with underlying respiratory diseases [24]), which may have been in part due to the possibility that 2 patients with negative BAL GM might have received voriconazole prior to diagnostic testing.
Interestingly, two patients, who met our criteria for IAA, were not or only briefly treated with antifungal therapy. Both patients survived. We cannot definitely exclude colonisation with Aspergillus because no histological examination was done. Elevated GM, long duration of invasive ventilation (11 and 15 days) and ICU LOS (17 and 29 days) argue in favour of IAA in these two patients. These two patients could have had a less severe form of IAA. Importantly, 8 of 9 patients had cultural evidence of Aspergillus spp., arguing against false-positive GM results. IAA is a relatively new field of research with few clinical studies and no prospective data. Because IAA patients do not fulfil EORTC criteria for invasive fungal disease [8] new diagnostic criteria were proposed [5, 22, 26]. These criteria do not allow for graduation of classification as with EORTC criteria (possible, probable, confirmed IA), have not been evaluated prospectively and diagnosis of IAA remains difficult. Furthermore, optimal treatment duration remains a matter of debate. IAA likely represents a heterogeneous entity and a benign course of disease could be possible although previous studies suggest otherwise with reported mortality of 33–47%, and 67% in Taiwan [7, 11, 26, 32, 36]. Mortality in our cohort was in concordance with previous reports in non-immunocompromised patients (33%) [26].
Our study has a few limitations. Firstly, it is limited by the retrospective design. We cannot rule out that cases of aspergillosis have been missed but the rarity of positive GM tests or growth of Aspergillus in cultures in the non-IAA group and the poor outcome in IAA which would lead to diagnostic work-up suggest otherwise. However, the true incidence of IAA might have been even higher. Secondly, two patients without IAA did not fulfil mycological IAA criteria but received long antifungal therapy for presumed IAA during hospitalisation and had extended LOS on the ICU. Therefore, differences concerning outcomes might have been underestimated between groups. Furthermore, the generalisability is limited by small sample size. However, patients’ characteristics and mortality are concordant with previous studies on IAA. Finally, owing to the retrospective design, it was not always possible to distinguish whether a complication was predisposing to IAA or a complication of IAA. Of note, both hospitals are the only major tertiary care centres in their area. Since all patients who fulfilled the listed criteria were included in the study, we consider recruitment bias unlikely.
In conclusion, IAA represents an underappreciated complication of influenza infection with severe morbidity and mortality in Swiss ICUs. Only 2 out of 16 Swiss ICUs participated in the study because screening and diagnosis of IAA were rarely done which is evidence for the poor awareness of this disease. While bacterial superinfection was frequent in influenza patients in the ICU, Aspergillus was even more common. The need for organ support therapy might serve as a predictor of IAA. Because of frequency and severity of disease, greater awareness of IAA is needed and lower thresholds for diagnostic testing (GM, bacterial and fungal cultures from BAL) and treatment should be implemented in the ICU, especially in patients requiring organ support therapies. A multicentre study including all university hospitals in Switzerland and two tertiary care centres is on its way to confirm our study outcome and raise awareness of this severe entity. Prospective studies are urgently needed to evaluate proposed diagnostic criteria, characterize clinical outcomes, identify patients at risk of IAA and define optimal antiviral and antifungal treatment and duration.
References
Abbott JD, Fernando HVJ, Gurling K, Meade BW (1952) Pulmonary aspergillosis following post-influenzal bronchopneumonia treated with antibiotics. BMJ 1:523–525. https://doi.org/10.1136/bmj.1.4757.523
Adalja AA, Sappington PL, Harris SP, Rimmele T, Kreit JW, Kellum JA, Boujoukos AJ (2011) Isolation of Aspergillus in three 2009 H1N1 influenza patients: flu and Aspergillus. Influenza Other Respir Viruses 5:225–229. https://doi.org/10.1111/j.1750-2659.2011.00202.x
ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS (2012) Acute respiratory distress syndrome: the Berlin definition. JAMA 307:2526–2533. https://doi.org/10.1001/jama.2012.5669
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R (2012) The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. https://doi.org/10.1164/ajrccm.149.3.7509706
Blot SI, Taccone FS, Van den Abeele A-M, Bulpa P, Meersseman W, Brusselaers N, Dimopoulos G, Paiva JA, Misset B, Rello J, Vandewoude K, Vogelaers D (2012) A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med 186:56–64. https://doi.org/10.1164/rccm.201111-1978OC
Cornet M, Mallat H, Somme D, Guérot E, Kac G, Mainardi JL, Fornes P, Gutmann L, Lavarde V (2003) Fulminant invasive pulmonary aspergillosis in immunocompetent patients—a two-case report. Clin Microbiol Infect 9:1224–1227. https://doi.org/10.1111/j.1469-0691.2003.00792.x
Crum-Cianflone NF (2016) Invasive aspergillosis associated with severe influenza infections. Open Forum Infect Dis 3:ofw171. https://doi.org/10.1093/ofid/ofw171
De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE (2008) Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46:1813–1821. https://doi.org/10.1086/588660
Gall J-RL, Lemeshow S, Saulnier F (1993) A new simplified acute physiology score (SAPS II) based on a European/North American Multicenter Study. JAMA 270:2957–2963. https://doi.org/10.1001/jama.1993.03510240069035
Garcia-Vidal C, Barba P, Arnan M, Moreno A, Ruiz-Camps I, Gudiol C, Ayats J, Orti G, Carratala J (2011) Invasive aspergillosis complicating pandemic influenza A (H1N1) infection in severely immunocompromised patients. Clin Infect Dis 53:e16–e19. https://doi.org/10.1093/cid/cir485
Ku Y-H, Chan K-S, Yang C-C, Tan C-K, Chuang Y-C, Yu W-L (2017) Higher mortality of severe influenza patients with probable aspergillosis than those with and without other coinfections. J Formos Med Assoc 116:660–670. https://doi.org/10.1016/j.jfma.2017.06.002
Lamoth F, Calandra T (2018) Let’s add invasive aspergillosis to the list of influenza complications. Lancet Respir Med 6:733–735. https://doi.org/10.1016/S2213-2600(18)30332-1
Lat A, Bhadelia N, Miko B, Furuya EY, Thompson GR (2010) Invasive aspergillosis after pandemic (H1N1) 2009. Emerg Infect Dis 16:971–973. https://doi.org/10.3201/eid1606.100165
Lionakis MS, Kontoyiannis DP (2003) Glucocorticoids and invasive fungal infections. Lancet 362:1828–1838. https://doi.org/10.1016/S0140-6736(03)14904-5
Martin-Loeches I, Schultz MJ, Vincent J-L, Alvarez-Lerma F, Bos LD, Solé-Violán J, Torres A, Rodriguez A (2017) Increased incidence of co-infection in critically ill patients with influenza. Intensive Care Med 43:48–58. https://doi.org/10.1007/s00134-016-4578-y
Martin-Loeches I, Lisboa T, Rhodes A, Moreno RP, Silva E, Sprung C, Chiche JD, Barahona D, Villabon M, Balasini C, Pearse RM, Matos R, Rello J, The ESICM H1N1 Registry Contributors (2011) Use of early corticosteroid therapy on ICU admission in patients affected by severe pandemic (H1N1)v influenza A infection. Intensive Care Med 37:272–283. https://doi.org/10.1007/s00134-010-2078-z
Matthay MA, Ware LB, Zimmerman GA (2012) The acute respiratory distress syndrome. J Clin Invest 122:2731–2740. https://doi.org/10.1172/JCI60331
McCullers JA (2014) The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol 12:252–262. https://doi.org/10.1038/nrmicro3231
Meersseman W, Lagrou K, Maertens J, Wijngaerden EV (2007) Invasive aspergillosis in the intensive care unit. Clin Infect Dis 45:205–216. https://doi.org/10.1086/518852
Nulens EF, Bourgeois MJ, Reynders MB (2017) Post-influenza aspergillosis, do not underestimate influenza B. Infect Drug Resist Vol 10:61–67. https://doi.org/10.2147/IDR.S122390
on behalf of the GETGAG Study Group, Moreno G, Rodríguez A, Reyes LF, Gomez J, Sole-Violan J, Díaz E, Bodí M, Trefler S, Guardiola J, Yébenes JC, Soriano A, Garnacho-Montero J, Socias L, del Valle OM, Correig E, Marín-Corral J, Vallverdú-Vidal M, Restrepo MI, Torres A, Martín-Loeches I (2018) Corticosteroid treatment in critically ill patients with severe influenza pneumonia: a propensity score matching study. Intensive Care Med 44:1470–1482. https://doi.org/10.1007/s00134-018-5332-4
Pagano L, Akova M, Dimopoulos G, Herbrecht R, Drgona L, Blijlevens N (2011) Risk assessment and prognostic factors for mould-related diseases in immunocompromised patients. J Antimicrob Chemother 66:i5–i14. https://doi.org/10.1093/jac/dkq437
Palmer LB, Greenberg HE, Schiff MJ (1991) Corticosteroid treatment as a risk factor for invasive aspergillosis in patients with lung disease. Thorax 46:15–20. https://doi.org/10.1136/thx.46.1.15
Prattes J, Flick H, Prüller F, Koidl C, Raggam RB, Palfner M, Eigl S, Buzina W, Zollner-Schwetz I, Thornton CR, Krause R, Hoenigl M (2014) Novel tests for diagnosis of invasive aspergillosis in patients with underlying respiratory diseases. Am J Respir Crit Care Med 190:922–929. https://doi.org/10.1164/rccm.201407-1275OC
Rice TW, Rubinson L, Uyeki TM, Vaughn FL, John BB, Miller RR, Higgs E, Randolph AG, Smoot BE, Thompson BT (2012) Critical illness from 2009 pandemic influenza A virus and bacterial coinfection in the United States. Crit Care Med 40:1487–1498. https://doi.org/10.1097/CCM.0b013e3182416f23
Schauwvlieghe AFAD, Rijnders BJA, Philips N, Verwijs R, Vanderbeke L, Van Tienen C, Lagrou K, Verweij PE, Van de Veerdonk FL, Gommers D, Spronk P, Bergmans DCJJ, Hoedemaekers A, Andrinopoulou E-R, van den Berg CHSB, Juffermans NP, Hodiamont CJ, Vonk AG, Depuydt P, Boelens J, Wauters J (2018) Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med 6:782–792. https://doi.org/10.1016/S2213-2600(18)30274-1
Schwartz IS, Friedman DZP, Zapernick L, Dingle TC, Lee N, Sligl W, Zelyas N, Smith SW (2020) High rates of influenza-associated invasive pulmonary aspergillosis may not be universal: a retrospective cohort study from Alberta, Canada. Clin Infect Dis ciaa007. https://doi.org/10.1093/cid/ciaa007
Shah MM, Hsiao EI, Kirsch CM, Gohil A, Narasimhan S, Stevens DA (2018) Invasive pulmonary aspergillosis and influenza co-infection in immunocompetent hosts: case reports and review of the literature. Diagn Microbiol Infect Dis 91:147–152. https://doi.org/10.1016/j.diagmicrobio.2018.01.014
Sheng Z-M, Chertow DS, Ambroggio X, McCall S, Przygodzki RM, Cunningham RE, Maximova OA, Kash JC, Morens DM, Taubenberger JK (2011) Autopsy series of 68 cases dying before and during the 1918 influenza pandemic peak. Proc Natl Acad Sci 108:16416–16421. https://doi.org/10.1073/pnas.1111179108
Shieh W-J, Blau DM, Denison AM, DeLeon-Carnes M, Adem P, Bhatnagar J, Sumner J, Liu L, Patel M, Batten B, Greer P, Jones T, Smith C, Bartlett J, Montague J, White E, Rollin D, Gao R, Seales C, Jost H, Metcalfe M, Goldsmith CS, Humphrey C, Schmitz A, Drew C, Paddock C, Uyeki TM, Zaki SR (2010) 2009 Pandemic Influenza A (H1N1). Am J Pathol 177:166–175. https://doi.org/10.2353/ajpath.2010.100115
Short KR, Kroeze EJBV, Fouchier RAM, Kuiken T (2014) Pathogenesis of influenza-induced acute respiratory distress syndrome. Lancet Infect Dis 14:57–69. https://doi.org/10.1016/S1473-3099(13)70286-X
van de Veerdonk FL, Kolwijck E, Lestrade PPA, Hodiamont CJ, Rijnders BJA, van Paassen J, Haas P-J, Oliveira dos Santos C, Kampinga GA, Bergmans DCJJ, van Dijk K, de Haan AFJ, van Dissel J, van der Hoeven HG, Verweij PE, Rahamat-Langendoen JC, Kullberg B-J, Netea MG, Brüggeman RJ, Hoedemaekers AW, Melchers WJG, Freudenburg W, Roescher N, Wiersinga WJ, van den Berg CHSB, Vonk AG, van Tienen C, van der Hoven B, van der Beek MT, Derde LPG, van Leer C, Aardema H, Lashof AO, Ang CW (2017) Influenza-associated aspergillosis in critically ill patients. Am J Respir Crit Care Med 196:524–527. https://doi.org/10.1164/rccm.201612-2540LE
Van de Veerdonk FL, Dewi I, Cunha C, van der Beek L (2018) Inhibition of host neuraminidase increases susceptibility to invasive pulmonary aspergillosis. OFID
Vanderbeke L, Spriet I, Breynaert C, Rijnders BJA, Verweij PE, Wauters J (2018) Invasive pulmonary aspergillosis complicating severe influenza: epidemiology, diagnosis and treatment. Curr Opin Infect Dis 1. https://doi.org/10.1097/QCO.0000000000000504
Ware LB, Matthay MA (2000) The acute respiratory distress syndrome. N Engl J Med 342:1334-1349. https://doi.org/10.1056/NEJM200005043421806
Wauters J, Baar I, Meersseman P, Meersseman W, Dams K, De Paep R, Lagrou K, Wilmer A, Jorens P, Hermans G (2012) Invasive pulmonary aspergillosis is a frequent complication of critically ill H1N1 patients: a retrospective study. Intensive Care Med 38:1761–1768. https://doi.org/10.1007/s00134-012-2673-2
Zhang Y, Sun W, Svendsen ER, Tang S, MacIntyre RC, Yang P, Zhang D, Wang Q (2015) Do corticosteroids reduce the mortality of influenza A (H1N1) infection? A meta-analysis. Crit Care 19:46. https://doi.org/10.1186/s13054-015-0764-5
Author information
Authors and Affiliations
Contributions
W.F. and A. W.C. had the idea and initiated the study and wrote the protocol. W. F., B. F., S. N., W. G. P. D., A. W. C., M. M. and S. J. managed the study and collected the data. W. F., K. G. R. and A. W. C. were responsible for and performed the statistical analysis. W. F., B. F., I. A., B. K., K. G. R., A. W. C., K. L. and J. P. interpreted the data. W. F., K. G. R. and A. W. C. drafted the manuscript. All authors amended and commented on the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 18 kb)
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Waldeck, F., Boroli, F., Suh, N. et al. Influenza-associated aspergillosis in critically-ill patients—a retrospective bicentric cohort study. Eur J Clin Microbiol Infect Dis 39, 1915–1923 (2020). https://doi.org/10.1007/s10096-020-03923-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-020-03923-7