Abstract
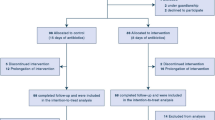
Literature for the treatment of hospitalized patients with community-acquired urinary tract infections (UTI) is limited. Previous outpatient studies do not support the use of oral beta-lactams compared with oral fluoroquinolones (FQ) due to poor clinical cure rates. However, recent studies evaluating intravenous (IV) beta-lactams in more complicated cases demonstrate promising cure rates. In addition, the use of more narrow-spectrum beta-lactams may be preferable when possible, due to a lower incidence of “collateral damage” compared with FQ. This was a retrospective, non-inferiority, single-center, cohort study evaluating the effectiveness of IV cefazolin compared with FQ for the treatment of community-acquired UTI in an inpatient setting. The primary endpoint was clinical failure, defined as the presence of one or more signs or symptoms of UTI that required a change in antibiotics, re-initiation of antibiotics for UTI treatment during the hospital stay, and re-hospitalization with a UTI diagnosis within 30 days after discharge. The secondary endpoints were a microbiological cure, hospital length of stay, inpatient antibiotic duration of treatment, emergence of resistance, and Clostridium difficile infection within 30 days of the end of antibiotic therapy. Overall, 73 patients were treated with either cefazolin (n = 43) or FQ (n = 30) between April 2015 to January 2016. The clinical failure rates were 2% and 7% in the cefazolin and FQ groups, respectively (p = 0.56). Additionally, there were no significant differences between the secondary endpoints. Treatment with cefazolin, a more narrow-spectrum agent with a potential for less “collateral damage,” was non-inferior to FQ for community-acquired UTI in an inpatient setting.

Similar content being viewed by others
References
Gupta K, Hooton TM, Naber KG et al (2011) International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52:e103–e120
Paterson D (2004) “Collateral damage” from cephalosporin or quinolone antibiotic therapy. Clin Infect Dis 38(Suppl. 4):S341–S345
Zervos M, Hershberger E, Nicolau DP et al (2003) Relationship between fluoroquinolone use and changes in susceptibility to fluoroquinolones of selected pathogens in 10 United States teaching hospitals, 1991–2000. Clin Infect Dis 37:1643–1648
Hooton TM, Scholes D, Gupta K, Stapleton AE, Roberts PL, Stamm WE (2005) Amoxicillin-clavulanate vs. ciprofloxacin for the treatment of uncomplicated cystitis in women: a randomized trial. JAMA 293:949–955
Hooton TM, Roberts PL, Stapleton AE (2012) Cefpodoxime vs. ciprofloxacin for short-course treatment of acute uncomplicated cystitis: a randomized trial. JAMA 307:583–589
Wagenlehner FM, Umeh O, Steenbergen J et al (2015) Ceftolazone-tazobactam compared with levofloxacin in treatment of complicated urinary-tract infections, including pyelonephritis: a randomized, double-blind, phase 3 trial (ASPECT-cUTI). Lancet 385:1949–1956
Hobbs AL, Shea KM, Daley MJ et al (2016) Are first-generation cephalosporins obsolete? A retrospective, non-inferiority, cohort study comparing empirical therapy with cefazolin versus ceftriaxone for acute pyelonephritis in hospitalized patients. J Antimicrob Cemother 71:1665–1671
Fasugba O, Gardner A, Mitchell BG, Mnatzaganian G (2015) Ciprofloxacin resistance in community- and hospital-acquired Escherichia coli urinary tract infections: a systematic review and meta-analysis of observational studies. BMC Infect Dis 15:545
FDA Drug Safety Communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects (2018). https://www.fda.gov/Drugs/DrugSafety/ucm511530.htm. Accessed 25 July 2018
Lipsky BA, Byren I, Hoey CT (2010) Treatment of bacterial prostatitis. Clin Infect Dis 50(12):1641–1652
Mody L, Juthani-Mehta M (2014) Urinary tract infections in older women: a clinical review. JAMA 311(8):844–854
Clinical and Laboratory Standards Institute (2016) Performance standards for antimicrobial susceptibility testing: twenty sixth informational supplement M100S. CLSI, Wayne, PA, USA, 2016
Acknowledgments
We acknowledge Stephanie Chiu, MPH for assistance with statistical analysis.
The contents of this publication were presented in a poster format at Eastern States Pharmacy Conference, Hershey, PA, USA, in May of 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This is a retrospective study that has been reviewed by the institutional review board prior (IRB) to the beginning of the study. A letter from the IRB has been attached in the supplementary section.
Informed consent
Informed consent was not required for this study as it was a retrospective study and no interventions were taken that would impact patient care. In addition, informed consent was not required according to per institutional policies and no identifying details were revealed regarding patients.
Transparency declarations
None to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Uppala, A., King, E. & Patel, D. Cefazolin versus fluoroquinolones for the treatment of community-acquired urinary tract infections in hospitalized patients. Eur J Clin Microbiol Infect Dis 38, 1533–1538 (2019). https://doi.org/10.1007/s10096-019-03582-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-019-03582-3