Abstract
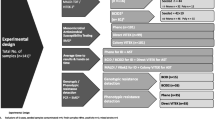
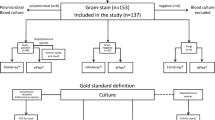
Early availability of microbiological results can improve treatment decisions of patients suffering from bloodstream infections. Direct inoculation of automated susceptibility testing (AST) platforms is an approach to shorten time-to-result in blood culture diagnostics. We performed a comparative evaluation of the two commercial AST systems VITEK®-2 and BD Phoenix™ for the direct inoculation with blood culture samples. Furthermore, two different methods of sample preparation were compared in this study. Positive blood cultures were prepared for direct inoculation by use of serum separator tubes and twofold centrifugation. AST was performed with the VITEK®-2 and the BD Phoenix™ system by the standard method according to the manufacturer’s recommendations using subcultures on solid media and by direct inoculation of blood culture samples. A hundred clinical samples from blood cultures were included in this study. Rapid AST by direct inoculation showed inter-test agreement rates ranging from 92.45 to 97.7%. Comparing both AST platforms, the VITEK®-2 system demonstrated a higher test accuracy for direct inoculation. No relevant difference was observed for the two different sample preparation methods. Direct inoculation is an easy and inexpensive approach to obtain early full panel phenotypic AST results in blood culture diagnostics. Sample preparation is sufficiently performed by a simple centrifugation method. Both commercial platforms, the VITEK®-2 and the BD Phoenix™, have proven suitable for the use of direct inoculation.
Similar content being viewed by others
References
Fleischmann C, Thomas-Rueddel DO, Hartmann M, Hartog CS, Welte T, Heublein S et al (2016) Hospital incidence and mortality rates of sepsis. Dtsch Arztebl Int 113(10):159–166
Martin GS (2012) Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes. Expert Rev Anti-Infect Ther 10(6):701–706
Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE et al (2009) Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 136(5):1237–1248
Garnacho-Montero J, Gutierrez-Pizarraya A, Escoresca-Ortega A, Fernandez-Delgado E, Lopez-Sanchez JM (2015) Adequate antibiotic therapy prior to ICU admission in patients with severe sepsis and septic shock reduces hospital mortality. Crit Care 19:302
Ferrer R, Martin-Loeches I, Phillips G, Osborn TM, Townsend S, Dellinger RP et al (2014) Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med 42(8):1749–1755
Tabak YP, Vankeepuram L, Ye G, Jeffers K, Gupta V, Murray PR (2018) Blood culture turnaround time in US acute care hospitals and implications for laboratory process optimization. J Clin Microbiol 56(12). https://doi.org/10.1128/JCM.00500-18
Liesenfeld O, Lehman L, Hunfeld KP, Kost G (2014) Molecular diagnosis of sepsis: new aspects and recent developments. Eur J Microbiol Immunol (Bp) 4(1):1–25
Sinha M, Jupe J, Mack H, Coleman TP, Lawrence SM, Fraley SI (2018) Emerging technologies for molecular diagnosis of sepsis. Clin Microbiol Rev 31(2). https://doi.org/10.1128/CMR.00089-17
Tan KE, Ellis BC, Lee R, Stamper PD, Zhang SX, Carroll KC (2012) Prospective evaluation of a matrix-assisted laser desorption ionization-time of flight mass spectrometry system in a hospital clinical microbiology laboratory for identification of bacteria and yeasts: a bench-by-bench study for assessing the impact on time to identification and cost-effectiveness. J Clin Microbiol 50(10):3301–3308
Maelegheer K, Nulens E (2017) Same-day identification and antibiotic susceptibility testing on positive blood cultures: a simple and inexpensive procedure. Eur J Clin Microbiol Infect Dis 36(4):681–687
Munoz-Davila MJ, Yague G, Albert M, Garcia-Lucas T (2012) Comparative evaluation of Vitek 2 identification and susceptibility testing of Gram-negative rods directly and isolated from BacT/ALERT-positive blood culture bottles. Eur J Clin Microbiol Infect Dis 31(5):663–669
Lupetti A, Barnini S, Castagna B, Capria AL, Nibbering PH (2010) Rapid identification and antimicrobial susceptibility profiling of Gram-positive cocci in blood cultures with the Vitek 2 system. Eur J Clin Microbiol Infect Dis 29(1):89–95
Wattal C, Oberoi JK (2016) Microbial identification and automated antibiotic susceptibility testing directly from positive blood cultures using MALDI-TOF MS and VITEK 2. Eur J Clin Microbiol Infect Dis 35(1):75–82
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R et al (2017) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 45(3):486–552
Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F et al (2015) 2015 ESC guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 36(44):3075–3128
Jorgensen JH (1993) Selection criteria for an antimicrobial susceptibility testing system. J Clin Microbiol 31(11):2841–2844
Tian Y, Zheng B, Wang B, Lin Y, Li M (2016) Rapid identification and multiple susceptibility testing of pathogens from positive-culture sterile body fluids by a combined MALDI-TOF mass spectrometry and Vitek susceptibility system. Front Microbiol 7:523
Bruins MJ, Bloembergen P, Ruijs GJ, Wolfhagen MJ (2004) Identification and susceptibility testing of Enterobacteriaceae and Pseudomonas aeruginosa by direct inoculation from positive BACTEC blood culture bottles into Vitek 2. J Clin Microbiol 42(1):7–11
Quesada MD, Gimenez M, Molinos S, Fernandez G, Sanchez MD, Rivelo R et al (2010) Performance of VITEK-2 Compact and overnight MicroScan panels for direct identification and susceptibility testing of Gram-negative bacilli from positive FAN BacT/ALERT blood culture bottles. Clin Microbiol Infect 16(2):137–140
Gherardi G, Angeletti S, Panitti M, Pompilio A, Di Bonaventura G, Crea F et al (2012) Comparative evaluation of the Vitek-2 Compact and Phoenix systems for rapid identification and antibiotic susceptibility testing directly from blood cultures of Gram-negative and Gram-positive isolates. Diagn Microbiol Infect Dis 72(1):20–31
Funke G, Funke-Kissling P (2004) Use of the BD PHOENIX Automated Microbiology System for direct identification and susceptibility testing of gram-negative rods from positive blood cultures in a three-phase trial. J Clin Microbiol 42(4):1466–1470
Wimmer JL, Long SW, Cernoch P, Land GA, Davis JR, Musser JM et al (2012) Strategy for rapid identification and antibiotic susceptibility testing of gram-negative bacteria directly recovered from positive blood cultures using the Bruker MALDI Biotyper and the BD Phoenix system. J Clin Microbiol 50(7):2452–2454
Prod’hom G, Durussel C, Greub G (2013) A simple blood-culture bacterial pellet preparation for faster accurate direct bacterial identification and antibiotic susceptibility testing with the VITEK 2 system. J Med Microbiol 62(Pt 5):773–777
Machen A, Drake T, Wang YF (2014) Same day identification and full panel antimicrobial susceptibility testing of bacteria from positive blood culture bottles made possible by a combined lysis-filtration method with MALDI-TOF VITEK mass spectrometry and the VITEK2 system. PLoS One 9(2):e87870
Romero-Gomez MP, Gomez-Gil R, Pano-Pardo JR, Mingorance J (2012) Identification and susceptibility testing of microorganism by direct inoculation from positive blood culture bottles by combining MALDI-TOF and Vitek-2 Compact is rapid and effective. J Inf Secur 65(6):513–520
Ling TK, Liu ZK, Cheng AF (2003) Evaluation of the VITEK 2 system for rapid direct identification and susceptibility testing of gram-negative bacilli from positive blood cultures. J Clin Microbiol 41(10):4705–4707
Lupetti A, Barnini S, Castagna B, Nibbering PH, Campa M (2010) Rapid identification and antimicrobial susceptibility testing of Gram-positive cocci in blood cultures by direct inoculation into the BD Phoenix system. Clin Microbiol Infect 16(7):986–991
Wisplinghoff H, Seifert H, Wenzel RP, Edmond MB (2003) Current trends in the epidemiology of nosocomial bloodstream infections in patients with hematological malignancies and solid neoplasms in hospitals in the United States. Clin Infect Dis 36(9):1103–1110
Karlowsky JA, Jones ME, Draghi DC, Thornsberry C, Sahm DF, Volturo GA (2004) Prevalence and antimicrobial susceptibilities of bacteria isolated from blood cultures of hospitalized patients in the United States in 2002. Ann Clin Microbiol Antimicrob 3:7
Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB (2004) Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39(3):309–317
Fritzenwanker M, Imirzalioglu C, Herold S, Wagenlehner FM, Zimmer KP, Chakraborty T (2018) Treatment options for carbapenem- resistant gram-negative infections. Dtsch Arztebl Int 115(20–21):345–352
Funding
The study was financed by internal funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study has been approved by the local research ethics committee (5358-11/17).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Höring, S., Massarani, A.S., Löffler, B. et al. Rapid antibiotic susceptibility testing in blood culture diagnostics performed by direct inoculation using the VITEK®-2 and BD Phoenix™ platforms. Eur J Clin Microbiol Infect Dis 38, 471–478 (2019). https://doi.org/10.1007/s10096-018-03445-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-018-03445-3