Abstract
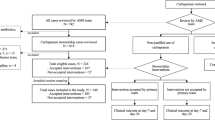
Antimicrobial stewardship programs are implemented to optimize the use of antibiotics and control the spread of antibiotic resistance. Many antimicrobial stewardship interventions have demonstrated significant efficacy in reducing unnecessary prescriptions of antibiotics, the duration of antimicrobial therapy, and mortality. We evaluated the benefits of a combination of rapid diagnostic tests and an active re-evaluation of antibiotic therapy 72 h after the onset of bloodstream infection (BSI). All patients with BSI from November 2015 to November 2016 in a 1100-bed university hospital in Rome, where an Infectious Disease Consultancy Unit (Unità di Consulenza Infettivologica, UDCI) is available, were re-evaluated at the bedside 72 h after starting antimicrobial therapy and compared to two pre-intervention periods: the UDCI was called by the ward physician for patients with BSI and the UDCI was called directly by the microbiologist immediately after a pathogen was isolated from blood cultures. Recommendations for antibiotic de-escalation or discontinuation significantly increased (54%) from the two pre-intervention periods (32% and 27.2%, p < 0.0001). Appropriate escalation also significantly increased (22.5%) from the pre-intervention periods (8.1% and 8.2%, p < 0.0001). The total duration of antibiotic therapy decreased with intervention (from 21.9 days [standard deviation, SD 15.4] in period 1 to 19.3 days [SD 13.3] in period 2 to 17.7 days in period 3 [SD 11.5]; p = 0.002) and the length of stay was significantly shorter (from 29.7 days [SD 29.3] in period 1 to 26.8 days [SD 24.7] in period 2 to 24.2 days in period 3 [SD 20.7]; p = 0.04) than in the two pre-intervention periods. Mortality was similar among the study periods (31 patients died in period 1 (15.7%), 39 (16.7%) in period 2, and 48 (15.3%) in period 3; p = 0.90). Rapid diagnostic tests and 72 h re-evaluation of empirical therapy for BSI significantly correlated with an improved rate of optimal antibiotic therapy and decreased duration of antibiotic therapy and length of stay.
Similar content being viewed by others
References
Diamantis S, Rioux C, Bonnal C et al (2012) Suitability of initial antibiotic therapy for the treatment of bloodstream infections and the potential role of antibiotic management teams in improving it. Eur J Clin Microbiol Infect Dis 31:1667–1671
Ly T, Gulia J, Pyrgos V, Waga M, Shoham S (2008) Impact upon clinical outcomes of translation of PNA FISH-generated laboratory data from the clinical microbiology bench to bedside in real time. Ther Clin Risk Manag 4:637–640
Carver PL, Lin SW, DePestel DD, Newton DW (2008) Impact of mecA gene testing and intervention by infectious disease clinical pharmacists on time to optimal antimicrobial therapy for Staphylococcus aureus bacteremia at a University Hospital. J Clin Microbiol 46:2381–2383
Holtzman C, Whitney D, Barlam T, Miller NS (2011) Assessment of impact of peptide nucleic acid fluorescence in situ hybridization for rapid identification of coagulase-negative staphylococci in the absence of antimicrobial stewardship intervention. J Clin Microbiol 49:1581–1582
Barlam TF, Cosgrove SE, Abbo LM et al (2016) Executive summary: implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 62:1197–1202
Barlam TF, Cosgrove SE, Abbo LM et al (2016) Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 62:e51–e77
Dunn K, O’Reilly A, Silke B, Rogers T, Bergin C (2011) Implementing a pharmacist-led sequential antimicrobial therapy strategy: a controlled before-and-after study. Int J Clin Pharm 33:208–214
Bosso JA, Drew RH (2011) Application of antimicrobial stewardship to optimise management of community acquired pneumonia. Int J Clin Pract 65:775–783
Slain D, Sarwari AR, Petros KO et al (2011) Impact of a multimodal antimicrobial stewardship program on Pseudomonas aeruginosa susceptibility and antimicrobial use in the intensive care unit setting. Crit Care Res Pract 2011:416426
Elligsen M, Walker SA, Pinto R et al (2012) Audit and feedback to reduce broad-spectrum antibiotic use among intensive care unit patients: a controlled interrupted time series analysis. Infect Control Hosp Epidemiol 33:354–361
Liew YX, Lee W, Tay D et al (2015) Prospective audit and feedback in antimicrobial stewardship: is there value in early reviewing within 48 h of antibiotic prescription? Int J Antimicrob Agents 45:168–173
Perez KK, Olsen RJ, Musick WL et al (2013) Integrating rapid pathogen identification and antimicrobial stewardship significantly decreases hospital costs. Arch Pathol Lab Med 137:1247–1254
Perez KK, Olsen RJ, Musick WL et al (2014) Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect 69:216–225
Clerc O, Prod’hom G, Vogne C, Bizzini A, Calandra T, Greub G (2013) Impact of matrix-assisted laser desorption ionization time-of-flight mass spectrometry on the clinical management of patients with Gram-negative bacteremia: a prospective observational study. Clin Infect Dis 56:1101–1107
Huang AM, Newton D, Kunapuli A et al (2013) Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis 57:1237–1245
Nguyen DT, Yeh E, Perry S et al (2010) Real-time PCR testing for mecA reduces vancomycin usage and length of hospitalization for patients infected with methicillin-sensitive staphylococci. J Clin Microbiol 48:785–790
Bauer KA, Perez KK, Forrest GN, Goff DA (2014) Review of rapid diagnostic tests used by antimicrobial stewardship programs. Clin Infect Dis 59(Suppl 3):S134–S145
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Fiori B, D’Inzeo T, Giaquinto A et al (2016) Optimized use of the MALDI BioTyper system and the FilmArray BCID panel for direct identification of microbial pathogens from positive blood cultures. J Clin Microbiol 54:576–584
Magiorakos AP, Srinivasan A, Carey RB et al (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281
Vlek AL, Bonten MJ, Boel CH (2012) Direct matrix-assisted laser desorption ionization time-of-flight mass spectrometry improves appropriateness of antibiotic treatment of bacteremia. PLoS One 7:e32589
Tan KE, Ellis BC, Lee R, Stamper PD, Zhang SX, Carroll KC (2012) Prospective evaluation of a matrix-assisted laser desorption ionization-time of flight mass spectrometry system in a hospital clinical microbiology laboratory for identification of bacteria and yeasts: a bench-by-bench study for assessing the impact on time to identification and cost-effectiveness. J Clin Microbiol 50:3301–3308
Banerjee R, Teng CB, Cunningham SA et al (2015) Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identification and susceptibility testing. Clin Infect Dis 61:1071–1080
Wong JR, Bauer KA, Mangino JE, Goff DA (2012) Antimicrobial stewardship pharmacist interventions for coagulase-negative staphylococci positive blood cultures using rapid polymerase chain reaction. Ann Pharmacother 46:1484–1490
Bork JT, Leekha S, Heil EL, Zhao L, Badamas R, Johnson JK (2015) Rapid testing using the Verigene Gram-negative blood culture nucleic acid test in combination with antimicrobial stewardship intervention against Gram-negative bacteremia. Antimicrob Agents Chemother 59:1588–1595
Tamma PD, Tan K, Nussenblatt VR, Turnbull AE, Carroll KC, Cosgrove SE (2013) Can matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF) enhance antimicrobial stewardship efforts in the acute care setting? Infect Control Hosp Epidemiol 34:990–995
Wenzler E, Goff DA, Mangino JE, Reed EE, Wehr A, Bauer KA (2016) Impact of rapid identification of Acinetobacter baumannii via matrix-assisted laser desorption ionization time-of-flight mass spectrometry combined with antimicrobial stewardship in patients with pneumonia and/or bacteremia. Diagn Microbiol Infect Dis 84:63–68
Cosgrove SE, Li DX, Tamma PD et al (2016) Use of PNA FISH for blood cultures growing Gram-positive cocci in chains without a concomitant antibiotic stewardship intervention does not improve time to appropriate antibiotic therapy. Diagn Microbiol Infect Dis 86:86–92
Nagel JL, Huang AM, Kunapuli A et al (2014) Impact of antimicrobial stewardship intervention on coagulase-negative Staphylococcus blood cultures in conjunction with rapid diagnostic testing. J Clin Microbiol 52:2849–2854
Box MJ, Sullivan EL, Ortwine KN et al (2015) Outcomes of rapid identification for gram-positive bacteremia in combination with antibiotic stewardship at a community-based hospital system. Pharmacotherapy 35:269–276
Hurst AL, Child J, Pearce K, Palmer C, Todd JK, Parker SK (2016) Handshake stewardship: a highly effective rounding-based antimicrobial optimization service. Pediatr Infect Dis J 35:1104–1110
Pfaller MA, Wolk DM, Lowery TJ (2016) T2MR and T2Candida: novel technology for the rapid diagnosis of candidemia and invasive candidiasis. Future Microbiol 11:103–117
Rödel J, Bohnert JA, Stoll S et al (2017) Evaluation of loop-mediated isothermal amplification for the rapid identification of bacteria and resistance determinants in positive blood cultures. Eur J Clin Microbiol Infect Dis 36:1033–1040
Ferrer R, Martin-Loeches I, Phillips G et al (2014) Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med 42:1749–1755
Schuts EC, Hulscher ME, Mouton JW et al (2016) Current evidence on hospital antimicrobial stewardship objectives: a systematic review and meta-analysis. Lancet Infect Dis 16:847–856
Okumura LM, Riveros BS, da Silva MMG, Veroneze I (2016) Strategies of hospital antimicrobial stewardship associated with different health outcomes. Lancet Infect Dis 16:999–1000
Boyles TH, Whitelaw A, Bamford C et al (2013) Antibiotic stewardship ward rounds and a dedicated prescription chart reduce antibiotic consumption and pharmacy costs without affecting inpatient mortality or re-admission rates. PLoS One 8:e79747
Ramsay C, Brown E, Hartman G, Davey P (2003) Room for improvement: a systematic review of the quality of evaluations of interventions to improve hospital antibiotic prescribing. J Antimicrob Chemother 52:764–771
Fraser GL, Stogsdill P, Dickens JD Jr, Wennberg DE, Smith RP Jr, Prato BS (1997) Antibiotic optimization. An evaluation of patient safety and economic outcomes. Arch Intern Med 157:1689–1694
Carling P, Fung T, Killion A, Terrin N, Barza M (2003) Favorable impact of a multidisciplinary antibiotic management program conducted during 7 years. Infect Control Hosp Epidemiol 24:699–706
Evans RS, Pestotnik SL, Classen DC et al (1998) A computer-assisted management program for antibiotics and other antiinfective agents. N Engl J Med 338:232–238
DiazGranados CA (2012) Prospective audit for antimicrobial stewardship in intensive care: impact on resistance and clinical outcomes. Am J Infect Control 40:526–529
Yeo CL, Chan DS, Earnest A et al (2012) Prospective audit and feedback on antibiotic prescription in an adult hematology-oncology unit in Singapore. Eur J Clin Microbiol Infect Dis 31:583–590
Acknowledgements
We are grateful to Leonida Passeri for his technical assistance in the data management.
Funding
No funding was received for this study. The present manuscript was revised by San Francisco Edit, which was supported by internal funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Ethical approval and informed consent
No ethical approval and no informed consent were required since the intervention is part of clinical routine activity.
Rights and permissions
About this article
Cite this article
Murri, R., Taccari, F., Spanu, T. et al. A 72-h intervention for improvement of the rate of optimal antibiotic therapy in patients with bloodstream infections. Eur J Clin Microbiol Infect Dis 37, 167–173 (2018). https://doi.org/10.1007/s10096-017-3117-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-017-3117-2