Abstract
The administration of neuraminidase inhibitors (NAIs) within 2 days after the onset of symptoms (early NAI therapy) has been shown to reduce mortality in adult patients with severe influenza. However, there is no sufficiently solid evidence supporting the effectiveness of early NAI therapy on mortality. We reviewed the clinical data from 506 adult patients who were hospitalized for influenza between March 2010 and March 2014, to investigate the impact of early NAI therapy on mortality. Nearly one-third of the study patients were infected with influenza B (influenza A, influenza B, and co-infection of both in 68.8%, 28.1%, and 3.2%, respectively), and were diagnosed using the polymerase chain reaction (PCR) method (33.6%). Less than half (233, 46.0%) had received early NAI therapy. Patients with early NAI therapy were admitted to the hospital earlier, more frequently infected with influenza A, and more frequently diagnosed using rapid influenza detection tests compared to those without early NAI therapy. Although patients without early NAI therapy presented with more serious clinical manifestations, such as an initial symptom of dyspnea, pneumonia, and intensive care unit admission, than those with early NAI therapy, the in-hospital mortality of the former (2.9%, 8/273) did not differ from that of the latter (3.4%, 8/233) (p = 0.75). We did not find a reduction in mortality associated with early NAI therapy in adult patients hospitalized for influenza. Further clinical studies including a large number of influenza B-infected patients with virus identification using PCR methodology rather than viral culture may be required to confirm the beneficial impact of early NAI therapy on mortality.
Similar content being viewed by others
Introduction
Neuraminidase inhibitors (NAIs) have been regarded as one of the most important therapeutic tools against influenza infection [1]. In adults with uncomplicated influenza, it has been reported that NAIs reduced symptom duration, prevented complications, and protected patients from hospitalization [2, 3]. For adults hospitalized with influenza or severe influenza in some cases, several observational studies reported that early administration of NAIs prevented fatal outcomes [4–8]. However, current evidence may not completely support the outstanding clinical effectiveness of NAIs. This has been questioned in a recent meta-analysis of adults with uncomplicated influenza, which was not sponsored by any pharmaceutical company [9]. The effectiveness of NAIs on clinical outcomes in adults with severe influenza has not been confirmed by any randomized controlled trial [10]. Although a recent meta-analysis which included data from more than 29,000 individual patients from 78 different centers admitted for H1N1 influenza during the 2009 pandemic showed a remarkable reduction in mortality for patients receiving NAIs [11], the study results were criticized due to methodological issues [12–15]. Therefore, further investigations into the clinical effectiveness of NAIs are still required, especially in adults hospitalized with influenza. We reviewed the clinical data from adults hospitalized with influenza to investigate the effectiveness of NAIs on their clinical outcomes.
Patients and methods
We identified adults (age ≥15 years) who had a positive result from diagnostic tests for influenza at Chung-Ang University Hospital (CAUH) between March 2010 and December 2013. From these patients, we selected those who were admitted to the hospital for the treatment of influenza and reviewed their medical records. We also included clinical data from adults hospitalized with influenza from our previous study [16], which was performed between January 2014 and March 2014 at four tertiary care centers, including CAUH. The following parameters were investigated: baseline characteristics including demographics and underlying disease or condition, and clinical characteristics including symptoms, treatment, and clinical outcomes. Based on these data, patients were included in the final study analysis only if: (1) they had available data on the time from the onset of symptoms to the administration of NAIs and (2) the time ranged from 0 to 14 days. Patients who received NAIs more than 14 days after the onset of symptoms were excluded because they were assumed to have inaccurately recalled the onset of symptoms. If a patient had experienced several episodes of influenza infection during the study period, only the first episode was included. Pneumonia was defined as the presence of a new or progressive infiltrate on chest radiograph plus two or more of the following symptoms or signs: fever, cough, sputum production, dyspnea, and an attending physician’s diagnosis of pneumonia. A patient was considered to have “pneumonia at presentation” or “early admission to the intensive care unit (ICU)” if he (or she) had pneumonia or was admitted to the ICU within one day after the beginning of the influenza evaluation, respectively. Early NAI therapy was defined as the administration of NAIs ≤2 days after the onset of symptoms. NAIs were administered as follows: one capsule (75 mg) of oseltamivir was taken twice a day and 300 mg of peramivir was injected once a day. This study was approved by the Institutional Review Board of each study hospital, including CAUH.
We compared the clinical characteristics of patients with early NAI therapy to those of patients without early NAI therapy. Continuous variables were compared using a Student’s t-test or the Mann–Whitney U-test. Categorical variables were compared using a χ2 test or Fisher’s exact test. A p-value of <0.05 was considered significant. Logistic regression analysis was performed to identify risk factors for in-hospital mortality. Statistical analyses were performed using SPSS software (version 18.0; SPSS, Chicago, IL, USA).
Results
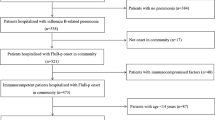
A total of 588 adults were hospitalized with influenza during the study period. Among these, 82 were excluded (16 received NAIs more than 14 days after the onset of symptoms, six were duplicates, 12 did not have available data on the time from the onset of symptoms to the administration of NAIs, and 48 had the occurrence of influenza infection 2 days after admission). Finally, 506 patients were included in study analyses.
The distribution of study patients according to each influenza season and the major circulating strain(s) during each period in the Republic of Korea are presented in Table 1 [17–20]. The characteristics for study patients are presented in Table 2. The median patient age was 58.5 years, and more than half of the patients were female (312, 61.7%). More than two-thirds were infected with influenza A (348, 68.8%), followed by influenza B (142, 28.1%) and both (16, 3.2%). Nearly one half of patients (232, 46.5%) had underlying diseases or conditions predisposing them to influenza complications. Pneumonia occurred in 143 patients (28.3%). Admission to the ICU was observed in 38 (7.5%). There were 443 patients (87.5%) who received NAIs and 233 (52.6% of 443) received early NAI therapy. The median duration of hospital admission was 4 days (ranging from 1 to 95 days). Sixteen patients (3.2%) died during admission and 10 deaths (2.0%) were assumed to be directly related to influenza infection. Deaths unrelated to influenza were caused by the development of hospital-acquired infections 2 weeks after influenza infection (n = 2), progression of malignancies (n = 1), acute myocardial infarction (n = 1), acute cerebral infarction (n = 1), and asphyxia (n = 1).
Patients who received early NAI therapy were compared to those without early NAI therapy (Table 2). The diagnosis of influenza by polymerase chain reaction (PCR) methodology and influenza B infection were both less commonly observed in patients who received early NAI therapy compared to those who did not. Patients with early NAI therapy were also admitted to the hospital earlier. Underlying chronic illnesses were similar in the two groups, except for chronic lung disease, which tended to be more common in patients without early NAI therapy, and recent chemotherapy, which was more common in patients with early NAI therapy. Patients without early NAI therapy had dyspnea, pneumonia, pneumonia at presentation, ICU admission, early ICU admission, and antibiotic therapy more frequently than patients who received early NAI therapy. The length of hospital admission was longer in patients without early NAI therapy. Both the in-hospital mortality rate and the influenza-related mortality rate were not different between the two groups.
The impact of early NAI therapy on the in-hospital mortality was evaluated in variable subgroups. Among influenza A-infected patients (n = 348), the in-hospital mortality rate for patients with early NAI therapy was lower compared to patients without NAI therapy [4/176 (2.3%) vs. 6/172 (3.5%)], although this difference was not statistically significant (p = 0.54). Among influenza B-infected patients (n = 142), the rate of patients with early NAI therapy (4/52, 7.7%) tended to be higher than in patients without early NAI therapy (1/90, 1.1%) (p = 0.06). In each subgroup of patients who had pneumonia at presentation (n = 131) and those who had early ICU admission (n = 33), in-hospital mortality rate of patients with early NAI therapy was not lower than that of patients without early NAI therapy [7.5% (3/40) vs. 8.8% (8/91) in the former subgroup and 66.7% (6/9) vs. 20.8% (5/24) in the latter subgroup].
Risk factors for in-hospital mortality were investigated using univariate and multivariate analyses. The univariate analyses showed that male gender, ICU admission, pneumonia, old age (age ≥65 years), chronic lung disease, congestive heart failure, dyspnea, positive bacterial culture, antibiotic therapy, oxygen supplementation, mechanical ventilation, and the use of vasoconstrictors were risk factors for in-hospital mortality. The multivariate analysis showed that ICU admission was the only risk factor for in-hospital mortality (adjusted odds ratio = 56.53, 95% confidence interval = 9.18–348.04, p < 0.001).
Discussion
Patients who received early NAI therapy had severe clinical manifestations such as an initial symptom of dyspnea, pneumonia, and ICU admission less frequently than those without early NAI therapy, and received a key intervention to reduce mortality earlier than patients without early NAI therapy. However, the in-hospital mortality rate in patients with early NAI therapy was not lower than that in those without. The multivariate analysis showed that early NAI therapy was not associated with in-hospital mortality. Thus, the findings from our cohort did not reveal any impact of early NAI therapy on mortality, which is not consistent with the results from previous studies [4–8, 11]. This discrepancy may be explained by a few characteristics of our study patients. First, unlike the previous studies [6–8, 11], influenza B infection was more frequently found (sole influenza B infection in 28.1% and co-infection of influenza A/B in 3.2%) in our patients. Several previous studies on severe influenza only investigated influenza A-infected patients [6, 8, 11] or the study was primarily composed of influenza A-infected patients [7]. A few clinical studies reported that the NAI, oseltamivir, was less effective against influenza B compared to influenza A [21, 22]. Although this finding has not been confirmed in further studies, it suggests that the inclusion of more influenza B-infected patients in our study may have affected outcomes due to early NAI therapy. This hypothesis could also be supported by the finding that in-hospital mortality was relatively frequent in influenza B-infected patients with early NAI therapy in this study; there was a tendency toward more frequent in-hospital mortality in influenza B-infected patients with early NAI therapy even compared to patients without early NAI therapy. Second, although two observational cohort studies which included a relatively large number of influenza B-infected patients (19% of 327 in one and 29% of 754 in the other) showed a reduction in mortality associated with early NAI therapy [4, 5], they did not utilize PCR methods for the diagnosis of influenza but used rapid influenza diagnostic tests and viral culture instead. Compared to the PCR methods used in our study, viral cultures have been reported to have a lower sensitivity, especially for samples with low viral loads [23]. Thus, only patients with relatively high viral loads may have been included in the two previous studies. Patients with low influenza virus-replicating activity in the respiratory tract may still present with severe influenza resulting from variable complications. If these patients were not included, the clinical effectiveness of early NAI therapy might be overestimated. We suggest that the impact of early NAI therapy on mortality should be further evaluated in studies including a large number of influenza B-infected patients using PCR methodology rather than viral culture to diagnose influenza.
The shorter length of hospital admission in patients with early NAI therapy should not be interpreted as a result of early NAI therapy in our cohort. Patients with early NAI therapy had pneumonia and ICU admission less frequently than patients without early NAI therapy, even from the early stage of their illnesses. But we could not determine which of the two—earlier NAI therapy and less severe initial presentation—affected the shorter length of hospital admission in patients with early NAI therapy.
This study has a few important limitations. First, laboratory investigation on the influenza virus, such as subtyping, quantification of viral loads, and detection of antiviral resistance to NAIs, was not performed. Second, we were not able to collect data on vaccinations due to the retrospective nature of this study.
In conclusion, we found that early NAI therapy did not affect mortality in adult patients hospitalized for influenza. Further studies including a large number of influenza B-infected patients using PCR methodology for diagnosis may be required to confirm the clinical benefit of early NAI therapy.
References
Harper SA, Bradley JS, Englund JA, File TM, Gravenstein S, Hayden FG, McGeer AJ, Neuzil KM, Pavia AT, Tapper ML, Uyeki TM, Zimmerman RK; Expert Panel of the Infectious Diseases Society of America (2009) Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 48:1003–1032
Kaiser L, Wat C, Mills T, Mahoney P, Ward P, Hayden F (2003) Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch Intern Med 163:1667–1672
Dobson J, Whitley RJ, Pocock S, Monto AS (2015) Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet 385:1729–1737
Lee N, Choi KW, Chan PKS, Hui DS, Lui GC, Wong BC, Wong RY, Sin WY, Hui WM, Ngai KL, Cockram CS, Lai RW, Sung JJ (2010) Outcomes of adults hospitalised with severe influenza. Thorax 65:510–515
McGeer A, Green KA, Plevneshi A, Shigayeva A, Siddiqi N, Raboud J, Low DE; Toronto Invasive Bacterial Diseases Network (2007) Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clin Infect Dis 45:1568–1575
Adisasmito W, Chan PKS, Lee N, Oner AF, Gasimov V, Aghayev F, Zaman M, Bamgboye E, Dogan N, Coker R, Starzyk K, Dreyer NA, Toovey S (2010) Effectiveness of antiviral treatment in human influenza A (H5N1) infections: analysis of a Global Patient Registry. J Infect Dis 202:1154–1160
Lee N, Leo YS, Cao B, Chan PK, Kyaw WM, Uyeki TM, Tam WW, Cheung CS, Yung IM, Li H, Gu L, Liu Y, Liu Z, Qu J, Hui DS (2015) Neuraminidase inhibitors, superinfection and corticosteroids affect survival of influenza patients. Eur Respir J 45:1642–1652
Hiba V, Chowers M, Levi-Vinograd I, Rubinovitch B, Leibovici L, Paul M (2011) Benefit of early treatment with oseltamivir in hospitalized patients with documented 2009 influenza A (H1N1): retrospective cohort study. J Antimicrob Chemother 66:1150–1155
Jefferson T, Jones MA, Doshi P, Del Mar CB, Hama R, Thompson MJ, Spencer EA, Onakpoya I, Mahtani KR, Nunan D, Howick J, Heneghan CJ (2014) Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev 4:CD008965
Hurt AC, Kelly H (2016) Debate regarding oseltamivir use for seasonal and pandemic influenza. Emerg Infect Dis 22:949–955
Muthuri SG, Venkatesan S, Myles PR et al (2014) Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza a H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med 2:395–404
Kmietowicz Z (2014) Study claiming Tamiflu saved lives was based on “flawed” analysis. BMJ 348:g2228
Nguyen-Van-Tam JS (2014) Principal author of PRIDE study responds to news story in The BMJ claiming that the study was based on “flawed” analysis. BMJ 348:g2935
Antes G, Meerpohl JJ (2014) Statistical and methodological concerns about the beneficial effect of neuraminidase inhibitors on mortality. Lancet Respir Med 2:e10
Leonardi-Bee J, Venkatesan S, Muthuri SG, Nguyen-Van-Tam JS, Myles PR; PRIDE research consortium (2014) Statistical and methodological concerns about the beneficial effect of neuraminidase inhibitors on mortality. Lancet Respir Med 2:e10–e12
Choi SH, Kim T, Park KH, Kwak YG, Chung JW, Lee MS (2017) Late diagnosis of influenza in adult patients during a seasonal outbreak. Korean J Intern Med (in press)
World Health Organization (WHO) (2011) Review of the 2010–2011 winter influenza season, northern hemisphere. Wkly Epidemiol Rec 86:222–227
World Health Organization (2012) Review of the 2011–2012 winter influenza season, northern hemisphere. Wkly Epidemiol Rec 87:233–240
World Health Organization (WHO) (2013) Review of the 2012–2013 winter influenza season, northern hemisphere. Wkly Epidemiol Rec 88:225–232
World Health Organization (WHO) (2014) Review of the 2013–2014 winter influenza season, northern hemisphere. Wkly Epidemiol Rec 89:245–256
Sugaya N, Mitamura K, Yamazaki M, Tamura D, Ichikawa M, Kimura K, Kawakami C, Kiso M, Ito M, Hatakeyama S, Kawaoka Y (2007) Lower clinical effectiveness of oseltamivir against influenza B contrasted with influenza A infection in children. Clin Infect Dis 44:197–202
Kawai N, Ikematsu H, Iwaki N, Satoh I, Kawashima T, Maeda T, Miyachi K, Hirotsu N, Shigematsu T, Kashiwagi S (2005) Factors influencing the effectiveness of oseltamivir and amantadine for the treatment of influenza: a multicenter study from Japan of the 2002–2003 influenza season. Clin Infect Dis 40:1309–1316
Kumar S, Henrickson KJ (2012) Update on influenza diagnostics: lessons from the novel H1N1 influenza a pandemic. Clin Microbiol Rev 25:344–361
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
There was no external funding for this work.
Conflict of interest
All authors have no conflicting interests to declare.
Ethical approval
The Medical Review Ethics Committee of each participating hospital approved the study.
Informed consent
Not required.
Rights and permissions
About this article
Cite this article
Choi, SH., Kim, T., Park, KH. et al. Early administration of neuraminidase inhibitors in adult patients hospitalized for influenza does not benefit survival: a retrospective cohort study. Eur J Clin Microbiol Infect Dis 36, 1673–1677 (2017). https://doi.org/10.1007/s10096-017-2982-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-017-2982-z