Abstract
Objective
Structural abnormalities in thalami and basal ganglia, in particular the globus pallidus (GP), are a neuroimaging hallmark of hereditary aceruloplasminemia (HA), yet few functional imaging data exit in HA carriers. This study investigated the iron-related structural and functional abnormalities in an Italian HA family.
Methods
Multimodal imaging was used including structural 3 T MRI, functional imaging (SPECT imaging with 123I-ioflupane (DAT-SPECT), cardiac 123I metaiodobenzylguanidine (123I-MIBG) scintigraphy, and 18F-fluorodeoxyglucose (18F-FDG)-PET imaging). In the proband, MRI and scintigraphic evaluations were performed at baseline, 2 and 4 years (structural imaging), and 2 years of follow-up period (functional imaging).
Results
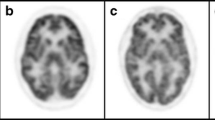
We investigated two cousins carrying a novel splicing homozygous mutation in intron 6 (IVS6 + 1 G > A) of CP gene. Interestingly, MRI features in both subjects were characterized by marked iron accumulation in the thalami and basal ganglia nuclei, while GP was not affected. MRI performed in the proband at 2 and 4 years of follow-up confirmed progressive neurodegeneration of the thalami and basal ganglia without the involvement of GP. Functional imaging showed reduced putaminal DAT uptake in both cousins, whereas cardiac MIBG and FDG uptakes performed in the proband were normal. Longitudinal scintigraphic investigations did not show significant changes over the time.
Conclusions
For HA carriers, our findings demonstrate that GP was spared by iron accumulation over the time. The nigrostriatal presynaptic dopaminergic system was damaged while the cardiac sympathetic system remained longitudinally preserved, thus expanding the imaging features of this rare inherited disorder.




Similar content being viewed by others
References
Miyajima H, Nishimura Y, Mizoguchi K, Sakamoto N, Shimizu T, Honda N (1987) Familial apoceruloplasmin deficiency associated with blepharospasm and retinal degeneration. Neurology 37:761–767
Yoshida K, Furihata K, Takeda S et al (1995) A mutation in the ceruloplasmin gene is associated with systemic hemosiderosis in humans. Nat Genet 9:267–272
Morita H, Ikeda S, Yamamoto K et al (1995) Hereditary ceruloplasmin deficiency with hemosiderosis: a clinicopathological study of a Japanese family. Ann Neurol 37:646–656
McNeill A, Birchall D, Hayflick SJ et al (2008) T2* and FSE MRI distinguishes four subtypes of neurodegeneration with brain iron accumulation. Neurology 70(18):1614–1619
Salsone M, Bagnato A, Novellino F et al (2009) Cardiac MIBG scintigraphy in primary progressive freezing gait. Parkinsonism Relat Disord 15(5):365–369
Sammarra I, Barbagallo G, Labate A et al (2019) Value of multimodal imaging approach to diagnosis of neurosarcoidosis. Brain Sci 9(10):243
Gelman N, Gorell JM, Barker PB et al (1999) MR imaging of human brain at 3.0 T: preliminary report on transverse relaxation rates and relation to estimated iron content. Radiology 210:759–767
Stelten BML, van Ommen W, Keizer K (2019) Neurodegeneration with brain iron accumulation: a novel mutation in the ceruloplasmin gene. JAMA Neurol 76(2):229–230
Zhou L, Chen Y, Li Y et al (2020) Intracranial iron distribution and quantification in aceruloplasminemia: a case study. Magn Reson Imaging 70:29–35
Watanabe M, Ohyama K, Suzuki M et al (2018) Aceruloplasminemia with abnormal compound heterozygous mutations developed neurological dysfunction during phlebotomy therapy. Intern Med 57(18):2713–2718
Kim HK, Ki CS, Kim YJ, Lee MS (2017) Radiological findings of two sisters with aceruloplasminemia presenting with chorea. Clin Neuroradiol 27(3):385–388
Haemers I, Kono S, Goldman S, Gitlin JD, Pandolfo M (2003) Clinical, molecular, and PET study of a case of aceruloplasminemia presenting with focal cranial dyskinesia. J Neurol Neurosurg Psychiatry 75:334–337
Suwijn SR, de Bruin K, de Bie RMA, Booij J (2014) The role of SPECT imaging of the dopaminergic system in translational research on Parkinson’s disease. Parkinsonism Relat Disord 20(Suppl 1):S184–S186
Sakamoto F, Shiraishi S, Ogasawara K et al (2020) A diagnostic strategy for Lewy body disease using DAT-SPECT, MIBG and combined index. Ann Nucl Med 34(6):415–423
Miyajima H, Takahashi Y, Kono S, Hishida A, Ishikawa K, Sakamoto M (2005) Frontal lobe dysfunction associated with glucose hypometabolism in aceruloplasminemia. J Neurol 252:996–997
Miyajima H, Takahashi Y, Kono S et al (2002) Glucose and oxygen hypometabolism in aceruloplasminemia brains. Intern Med 41:186–190
Fasano A, Colosimo C, Miyajima H, Tonali PA, Re TJ, Bentivoglio AR (2008) Aceruloplasminemia: a novel mutation in a family with marked phenotypic variability. Mov Disord 23(5):751–755
Cuenca MC, Marchi G, Barqué A, Esteban-Jurado C, Marchetto A, Giorgetti A et al (2020) Genetic and clinical heterogeneity in thirteen new cases with aceruloplasminemia. Atypical anemia as a clue for an early diagnosis. Int J Mol Sci 21(7):2374
Yazaki M, Yoshida K, Nakamura A et al (1998) A novel splicing mutation in the ceruloplasmin gene responsible for hereditary ceruloplasmin deficiency with hemosiderosis. J Neurol Sci 156(1):30–34
Hatanaka Y, Okano T, Oda K, Yamamoto K, Yoshida K (2003) Aceruloplasminemia with juvenile-onset diabetes mellitus caused by exon skipping in the ceruloplasmin 1. Intern Med 42(7):599–604
Acknowledgements
The authors are grateful to the two patients that kindly participated in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All procedures performed in the study involving human participant were in accordance with the ethical standards of the Institutional Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Written informed consent was obtained from both participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salsone, M., Arabia, G., Annesi, G. et al. Aceruloplasminemia: a multimodal imaging study in an Italian family with a novel mutation. Neurol Sci 43, 1791–1797 (2022). https://doi.org/10.1007/s10072-021-05613-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05613-4