Abstract
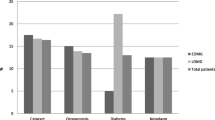
The study evaluated the efficacy of low-dose tacrolimus for treating Myasthenia Gravis (MG). Data were collected from 97 patients treated with low-dose tacrolimus from February 2011 to April 2015. Metabolic analysis was performed to determine more accurate tacrolimus dosing and patients were followed-up within clinic every 6 months for up to 4 years. The myasthenia gravis-specific activities of daily living scale was used to assess MG symptoms and their effects on patients’ daily activities. All side effects and adverse reactions were thoroughly documented. At the end of follow-up, 6 patients were in complete stable remission, 17 patients were in pharmacological remission, 26 patients were in minimal manifestation status, 32 patients were improved, 2 patients were unchanged, 11 patients had worsening symptoms, and 3 patients died. Side effects were reported and/or observed in 24 patients, of which 7 patients experienced elevated blood glucose, 2 patients developed neoplasms, 3 patients developed gastrointestinal symptoms, 3 showed mild increases in aminotransferases, 3 patients suffered from bone marrow suppression, 2 patients suffered from skin rashes and erythema, and 1 patient required discontinuation of therapy. Transient renal insufficiency was also observed in 1 patient and 3 other patients had minor miscellaneous side effects. This study adds some knowledge on the efficacy and side effects of low-dose tacrolimus in the treatment of MG. Tacrolimus immunotherapy is a valid option for the management of MG, and can be gradually reduced in dose once symptoms are improved until complete withdrawal is achieved.


Similar content being viewed by others
References
Cruz JL, Wolff ML, Vanderman AJ, Brown JN (2015) The emerging role of tacrolimus in Myasthenia Gravis. Ther Adv Neurol Disord 8:92–103
Wolfe GI, Herbelin L, Nations SP, Foster B, Bryan WW, Barohn RJ (1999) Myasthenia gravis activities of daily living profile. Neurology 52:1487–1489
Minami N, Fujiki N, Doi S (2011) Five-year follow-up with low-dose tacrolimus in patients with myasthenia gravis. J Neurol Sci 300:59–62
Thomson AW, Bonham CA, Zeevi A (1995) Dec mode of action of tacrolimus (FK506): molecular and cellular mechanisms. Ther Drug Monit 17(6):584–591 (review)
Meekins GD, So Y, Quan D (2008) American association of neuromuscular & electrodiagnostic medicine evidenced-based review: use of surface electromyography in the diagnosis and study of neuromuscular disorders. Muscle Nerve 38:1219–1224
Jaretzki A 3rd, Barohn RJ, Ernstoff RM (2000) Myasthenia gravis: recommendations for clinical research standards. Task force of the medical scientific advisory board of the myasthenia gravis foundation of america. Ann Thorac Surg 70:327–334
Evoli A, Di Schino C, Marsili F, Punzi C (2002) Successful treatment of myasthenia gravis with tacrolimus. Muscle Nerve 25:111–114
Konishi T, Yoshiyama Y, Takamori M (2003) Clinical study of fk506 in patients with myasthenia gravis. Muscle Nerve 28:570–574
Zhao CB, Zhang X, Zhang H (2011) Clinical efficacy and immunological impact of tacrolimus in chinese patients with generalized myasthenia gravis. Int Immunopharmacol 11:519–524
Nagane Y, Utsugisawa K, Obara D, Kondoh R, Terayama Y (2005) Efficacy of low-dose fk506 in the treatment of myasthenia gravis–a randomized pilot study. Eur Neurol 53:146–150
Ponseti JM, Azem J, Fort JM (2005) Long-term results of tacrolimus in cyclosporine-and prednisone-dependent myasthenia gravis. Neurology 64:1641–1643
Ponseti JM, Gamez J, Azem J, Lopez-Cano M, Vilallonga R, Armengol M (2008) Tacrolimus for myasthenia gravis: a clinical study of 212 patients. Ann N Y Acad Sci 1132:254–263
Glowacki F, Lionet A, Hammelin JP (2011) Influence of cytochrome p450 3a5 (cyp3a5) genetic polymorphism on the pharmacokinetics of the prolonged-release, once-daily formulation of tacrolimus in stable renal transplant recipients. Clin Pharmacokinet 50:451–459
Op den Buijsch RA, Christiaans MH, Stolk LM et al (2007) Tacrolimus pharmacokinetics: influence of adenosine triphosphate-binding cassette b1 (abcb1) and cytochrome (cyp) 3a polymorphisms. Fundam Clin Pharmacol 21:427–435
Ferraresso M, Tirelli A, Ghio L et al (2007) Influence of the cyp3a5 genotype on tacrolimus pharmacokinetics and pharmacodynamics in young kidney transplant recipients. Pediatr Transplant 11:296–300
Xie HG, Wood AJ, Kim RB, Stein CM, Wilkinson GR (2004) Genetic variability in cyp3a5 and its possible consequences. Pharmacogenomics 5:243–272
Tada M, Shimohata T, Tada M, et al (2006) Long-term therapeutic efficacy and safety of low-dose tacrolimus (fk506) for Myasthenia Gravis. J Neurol Sci 247:17–20
Tada M, Shimohata T, Tada M, et al (2006) Long-term therapeutic efficacy and safety of low-dose tacrolimus (fk506) for Myasthenia Gravis. J Neurol Sci 247:17–20.
Furukawa Y, Yoshikawa H, Iwasa K, Yamada M (2008) Clinical efficacy and cytokine network-modulating effects of tacrolimus in Myasthenia Gravis. J Neuroimmunol 195:108–115
Drachman DB, Jones RJ, Brodsky RA (2003) Treatment of refractory myasthenia: “rebooting” with high-dose cyclophosphamide. Ann Neurol 53:29–34
Acknowledgements
This study was supported by the by 309th Hospital of the Chinese PLA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The appropriate ethics review board approved the design of this.
Conflict of interest
The authors report no conflicts of interest.
Rights and permissions
About this article
Cite this article
Tao, X., Wang, W., Jing, F. et al. Long-term efficacy and side effects of low-dose tacrolimus for the treatment of Myasthenia Gravis. Neurol Sci 38, 325–330 (2017). https://doi.org/10.1007/s10072-016-2769-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-016-2769-5