Abstract
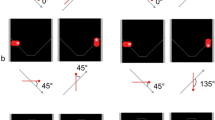
The ability to perceive biological motion (BM) has been demonstrated in a number of species including humans but the few studies of non-human primates have been relatively inconclusive. We investigated whether common marmosets (Callithrix jacchus) are able to perceive biological motion, using a novel method to test non-human primates. Marmosets (7 male and 7 female) were trained to remove a cover from a container and look inside it, revealing a computer screen. Then they were presented with images on this computer screen consisting of a novel BM pattern (a walking hen) and 4 manipulations of that pattern (a static frame of this pattern and inverted, scrambled, and rotating versions of the pattern). The behavioural responses of the marmosets were recorded and used to assess discrimination between stimuli. BM was attended to by females but not males, as shown by active inspection behaviour, mainly movement of the head towards the stimulus. Females paid significantly less attention to all of the other stimuli. This indicates the females’ ability to attend to biological motion. Females showed slightly more attention to the inverted BM than to the static, scrambled, and rotating patterns. The males were less attentive to all of the stimuli than were the females and, unlike the females, responded to all stimuli in a similar manner. This sex difference could be due to an inability of males to recognise BM altogether or to a lesser amount of curiosity. Considered together with the findings of previous studies on chicks and humans, the results of the present study support the notion of a common mechanism across species for the detection of BM.




Similar content being viewed by others
References
Bachevalier J, Hagger C, Bercu BB (1989) Gender differences in visual habit formation in 3-month-old rhesus monkeys. Dev Psychobio 22:585–599
Bertenthal BI, Proffitt DR, Spetner NB, Thomas MA (1985) The development of infant sensitivity to biomechanical motions. Child Dev 56:531–543
Bertenthal BI, Proffitt DR, Kramer SJ (1987) Perception of biomechanical motions by infants: implementation of various processing constraints. J Exp Psychol Hum Percept Perform 13(4):577–585
Blake R (1993) Cats perceive biological motion. Psychol Sci 4(1):54–57
Booth AE, Bertenthal BI, Pinto J (2002) Perception of the symmetrical patterning of human gait by infants. Dev Psychol 38(4):554–563
Brosnan MJ, Scott FJ, Fox S, Pye J (2004) Gestalt processing in autism: failure to process perceptual relationships and the implications for contextual understanding. J Child Psychol Psychiatry 45:459–469
Caine NG, Mundy NI (2000) Demonstration of a foraging advantage for trichromatic marmosets (Callithrix geoffroyi) dependent on food colour. Proc R Soc Lond B 267:439–444
Clara E, Regolin L, Vallortigara G, Rogers L (2007) Perception of the stereokinetic illusion by the common marmoset (Callithrix jacchus). Anim Cogn 10:135–140
Dittrich WH, Lea SEG, Barrett J, Gurr PR (1998) Categorisation of natural movements by pigeons: visual concept discrimination and biological motion. J Exp Anal Behav 70(3):281–299
Fox R, McDaniel C (1982) The perception of biological motion by human infants. Science 218(29):486–487
Itani J (1958) On the acquisition and propagation of a new food habit in the natural group of the Japanese monkey at Takasaki-yama. Primates 1:84–98
Jacobs GH (1998) A perspective on color vision in platyrrhine monkeys. Vis Res 38:3307–3313
Johansson G (1973) Visual perception of biological motion and a model for its analysis. Percept Psychophys 14(2):201–211
Kaplan G, Rogers LJ (1999) Parental care in marmosets (Callithrix jacchus jacchus): development and effect of anogenital licking on exploration. J Comp Psychol 113:269–276
Kaplan G, Rogers LJ (2006) Head-cocking as a form of exploration in the common marmoset and its development. Dev Psychobio. doi:10.1002/dev.20155:551-560
Kappeler PM (1987) The acquisition process of a novel behaviour pattern in a group of ring-tailed lemurs (Lemur catta). Primates 28:225–228
Kawai M (1963) On the newly acquired behaviours of the natural troop of Japanese monkeys on Koshima Island. Primates 4:113–115
Kawai M (1965) Newly acquired pre-cultural behaviour of the natural troop of Japanese monkeys on Koshima Islet. Primates 6:1–30
Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W (2009) Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature 459:257–261
Lazaro-Perea C (2001) Intergroup interactions in wild common marmosets, Callithrix jacchus: territorial defence and assessment of neighbours. Anim Behav 62:11–21
Mather G, West S (1993) Recognition of animal locomotion from dynamic point-light displays. Percept 22:759–766
Meary D, Kitromilides E, Mazens K, Graff C, Gentaz E (2007) Four-day-old human neonates look longer at non-biological motion of a single point-of-light. PLoS ONE. doi:10.1371/journal.pone.0000186
Neiworth JJ, Gleichman AJ, Olinick AS, Lamp KE (2006) Global and local processing in adult humans (Homo sapiens), 5-year old children (Homo sapiens), and adult cotton top tamarins (Saguinus oedipus). J Comp Psychol 120(4):323–330
Omori E (1997) Comparative study of visual perception using Johansson’s stimuli. In: Chase S, Watanabe S (eds) Pattern recognition in humans and animals. Keio University, Tokyo, pp 27–30
Omori E, Watanabe S (1996) Discrimination of Johansson’s stimuli in pigeons. Int J Comp Psychol 9:92
Oram MW, Perrett PI (1994) Responses of anterior superior temporal polysensory (STPa) neurons to “biological motion” stimuli. J Cogn Neurosci 6(2):99–116
Parron C, Deruelle C, Fagot J (2007) Processing of biological motion point-light displays by baboons (Papio papio). J Exp Psychol Anim Behav Process 33(4):381–391
Pavlova M, Krageloh-Mann I, Sokolov A, Birbaumer N (2001) Recognition of point-light biological motion displays by young children. Percept 30(8):925–933
Regolin L, Tommasi L, Vallortigara G (2000) Visual perception of biological motion in newly hatched chicks as revealed by an imprinting procedure. Anim Cogn 3:53–60
Rogers LJ, Stafford D, Ward JP (1993) Head cocking in galagos. Anim Behav 45:943–952
Rouff JH, Sussman RW, Strube MJ (2005) Personality traits in captive lion-tailed macaques (Macaca silenus). Am J Primatol 67:177–198
Saito A, Mikamki A, Kawamura S, Ueno Y, Hiramatsu C, Widayati KA, Suryobroto B, Teramoto M, Mori Y, Nagano K, Fujita K, Kuroshima H, Hesagawa T (2005) Advantages of Dichromats over Trichromats in discrimination of colour-camouflaged stimuli in non-human primates. Am J Primatol 67:425–436
Simion F, Regolin L, Bulf H (2008) A predisposition for biological motion in the newborn baby. Proc National Acad Sci 105(2):809–813
Tomonaga M (2001) Visual search for biological motion patterns in chimpanzees (Pan troglodytes). Psychologia 44:46–59
Troje NF (2003) Reference frames for orientation anisotropies in face recognition and biological-motion perception. Percept 32:201–210
Troje NF, Westhoff C (2006) The inversion effect in biological motion perception: evidence for a “life detector”. Curr Biol 16:821–824
Vallortigara G, Regolin L (2006) Gravity bias in the interpretation of biological motion by inexperienced chicks. Curr Biol 16(8):R279–R280
Vallortigara G, Regolin L, Marconato F (2005) Visually inexperienced chicks exhibit spontaneous preference for biological motion. PLoS Biology. doi:10.1317/journal.pbio.0030208
Vallortigara G, Snyder A, Kaplan G, Bateson PPG, Clayton NS, Rogers LJ (2008) Are animals autistic savants? PLoS Biology 6:208–214
Visalberghi E, Janson CH, Agostini I (2003) Response toward novel foods and novel objects in wild Cebus paella. Int J Primatol 24(3):653–675
Yamaguchi MK, Fujita K (1999) Perception of biological motion by newly hatched chicks and quail. Percept 28:23–24
Yamamoto ME, Domeniconi C, Box H (2004) Sex differences in common marmosets (Callithrix jacchus) in response to an unfamiliar food task. Folia Primatol 45:249–254
Acknowledgments
We are grateful to the Australian Research Council for funding to L.J.R. in support of the marmoset colony at UNE. This project was part of the requirements of J.B.’s Honours degree at the University of New England. The housing and testing conditions of the marmosets were in accordance with the principles and regulations of the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (1997) and approved by the Animal Ethics committee at the University of New England (AEC08/037).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
S1. The walking hen: This sequence was obtained by locating points of light on the digitalised version of a video recording of a real walking hen. It shows the hen walking towards the left side of the screen. (AVI 14 kb)
S2. The inverted hen: This stimulus was obtained by inverting the walking hen from a canonical upright position to an upside down position. (AVI 393 kb)
S3. The rotating hen: To obtain the rotating hen, a single frame of the walking hen sequence was rotated rigidly anticlockwise as if it were a solid object. (AVI 13 kb)
S4. The scrambled hen: To obtain the scrambled hen, the position of each point of light of the walking hen sequence was displaced, resulting in a motion that is still perceived by humans as being biological, though not belonging to any particular known species. (AVI 13 kb)
Rights and permissions
About this article
Cite this article
Brown, J., Kaplan, G., Rogers, L.J. et al. Perception of biological motion in common marmosets (Callithrix jacchus): by females only. Anim Cogn 13, 555–564 (2010). https://doi.org/10.1007/s10071-009-0306-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-009-0306-0