Abstract
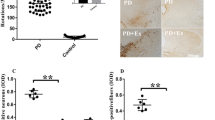
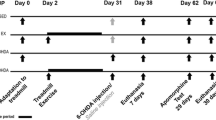
Physical function-improving effects of a silk amino acid preparation (SAA) in Parkinson’s disease (PD) model rats were investigated. 6-Hydroxydopamine (6-OHDA, 8 μg)+ascorbic acid (0.6 μg) was injected into right medial forebrain bundle of 8-week-old Sprague-Dawley rats to induce PD, and SAA (50, 160, or 500 mg/kg) was orally administered for 30 days. On day 15 and 30, behavioral abnormalities, neuronal loss, and dopamine and its metabolites were analyzed. Injection of 6-OHDA impaired pole test performances, which were markedly improved by treatment with SAA. Increased using rate of ipsilateral (normal) forelimb in cyclinder test and apomorphine (0.05 mg/kg)-induced circling behavior of PD rats were remarkably corrected by the compounds. In addition, 6-OHDA-induced loss of neurons as well as decreases in dopamine and its metabolites were significantly attenuated by SAA. The results indicate that SAA preserves movement function of PD model animals by protecting dopamine neurons against 6-OHDA neurotoxicity.
Article PDF
Similar content being viewed by others
References
Alexi T, Borlongan CV, Faull RL, Williams CE, Clark RG, Gluckman PD, Hughes PE. Neuroprotective strategies for basal ganglia degeneration: Parkinson’s and Huntington’s diseases. Prog. Neurobiol. 60: 409–470 (2000)
Agid Y. Parkinson’s disease: Pathophysiology. Lancet 337: 1321–1324 (1991)
Olsson M, Nikkhah G, Bentlage C, Bjarklund A. Forelimb akinesia in the rat Parkinson model: Differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J. Neurosci. 15: 3863–3875 (1995)
Abe K, Taguchi K, Wasai T, Ren J, Utsunomiya I, Shinohara T, Miyatake T, Sano T. Biochemical and pathological study of endogenous 1-benzyl-1,2,3,4-tetrahydroisoquinoline-induced parkinsonism in the mouse. Brain Res. 907: 134–138 (2001)
Kim SU, Park IH, Kim TH, Kim KS, Choi HS, Hong SH, Bang JH, Lee MA, Joo IS, Lee CS, Kim YS. Brain transplantation of human neural stem cells transduced with tyrosine hydroxylase and GTP cyclohydrolase 1 provides functional improvement in animal models of Parkinson disease. Neuropathology 26: 129–140 (2006)
Yasuhara T, Matsukawa N, Hara K, Yu G, Xu L, Maki M, Kim SU, Borlongan CV. Transplantation of human neural stem cells exerts neuroprotection in a rat model of Parkinson’s disease. J. Neurosci. 26: 12497–12511 (2006)
Redmond DE Jr, Bjugstad KB, Teng YD, Ourednik V, Ourednik J, Wakeman DR, Parsons XH, Gonzalez R, Blanchard BC, Kim SU, Gu Z, Lipton SA, Markakis EA, Roth RH, Elsworth JD, Sladek JR Jr, Sidman RL, Snyder EY. Behavioral improvement in a primate Parkinson’s model is associated with multiple homeostatic effects of human neural stem cells. P. Natl. Acad. Sci. USA 104: 12175–12180 (2007)
Jeon S, Kim YJ, Kim S-T, Moon W, Chae Y, Kang M, Chung M-Y, Lee H, Hong M-S, Chung J-H, Joh TH, Lee H, Park H-J. Proteomic analysis of the neuroprotective mechanisms of acupuncture treatment in a Parkinson’s disease mouse model. Proteomics 8: 4822–4832 (2008)
Borah A, Mohanakumar KP. Melatonin inhibits 6-hydroxydopamine production in the brain to protect against experimental parkinsonism in rodents. J. Pineal Res. 47: 293–300 (2009)
Szczudlik A, Rudziñska M. Neuroprotective effect of dopamine agonists. Neurol. Neurochir. Pol. 41: S22–S28 (2007)
Schapira AH. Molecular and clinical pathways to neuroprotection of dopaminergic drugs in Parkinson disease. Neurology 72: S44–S50 (2009)
Kondo T. Levodopa therapy from the neuroprotection viewpoint. From a clinical outlook. J. Neurol. 252(Suppl. 4): IV/32–IV/36 (2005)
Lang AE, Lozano AM. Parkinson’s disease. Second of two parts. New Engl. J. Med. 339: 1130–1143 (1998)
Liu WG, Chen Y, Li B, Lu GQ, Chen SD. Neuroprotection by pergolide against levodopa-induced cytotoxicity of neural stem cells. Neurochem. Res. 29: 2207–2214 (2004)
Ogawa N, Asanuma M, Miyazaki I, Diaz-Corrales FJ, Miyoshi K. L-DOPA treatment from the viewpoint of neuroprotection. Possible mechanism of specific and progressive dopaminergic neuronal death in Parkinson’s disease. J. Neurol. 252(Suppl. 4): IV/23–IV/31 (2005)
Agnati LF, Leo G, Vergoni AV, Martínez E, Hockemeyer J, Lluis C, Franco R, Fuxe K, Ferré S. Neuroprotective effect of L-DOPA coadministered with the adenosine A2A receptor agonist CGS 21680 in an animal model of Parkinson’s disease. Brain Res. Bull. 64: 155–164 (2004)
Wu WR, Zhu XZ. Involvement of monoamine oxidase inhibition in neuroprotective and neurorestorative effects of Ginkgo biloba extract against MPTP-induced nigrostriatal dopaminergic toxicity in C57 mice. Life Sci. 65: 157–164 (1999)
Levites Y, Weinreb O, Maor G, Youdim MBH, Mandel S. Green tea polyphenol (-)-epigallocatechin-3-gallate prevents N-methyl-4-phenyl-1,2,3,6-terahydropyridine-induced dopaminergic neurodegeneration. J. Neurochem. 78: 1073–1082 (2001)
Van Kampen J, Robertson H, Hagg T, Drobitch R. Neuroprotective actions of the ginseng extract G115 in two rodent models of Parkinson’s disease. Exp. Neurol. 184: 521–529 (2003)
Rojas P, Serrano-García N, Mares-Sámano JJ, Medina-Campos ON, Pedraza-Chaverri J, Ogren SO. EGb761 protects against nigrostriatal dopaminergic neurotoxicity in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism in mice: Role of oxidative stress. Eur. J. Neurosci. 28: 41–50 (2008)
Lee KG, Yeo JH, Lee YW, Kweon HY, Woo SO, Han SM, Kim JH. Studies on industrial utilization of silk protein. Korean J. Food Ind. 36: 25–37 (2003)
Shin M, Park M, Youn M, Lee Y, Nam M, Park I, Jeong Y. Effects of silk protein hydrolysates on blood glucose and serum lipid in db/db diabetic mice. J. Korean Soc. Food Sci. Nutr. 35: 1343–1348 (2006)
Lee Y, Park M, Choi J, Kim J, Nam M, Jeong Y. Effects of silk protein hydrolysates on blood glucose level, serum insulin, and leptin secretion in OLEFT rats. J. Korean Soc. Food Sci. Nutr. 36: 703–707 (2007)
Hyun CK, Kim IY, Frost SC. Soluble fibroin enhances insulin sensitivity and glucose metabolism in 3T3-L1 adipocytes. J. Nutr. 134: 3257–3263 (2004)
Hwang E, Kang B, Kim B, Lee HJ. Protein quality evaluation and effect of plasma contents of acid hydrolysates of cocoon in rats fed by high cholesterol, high triglyceride, and high sucrose diet. J. Korean Soc. Food Sci. Nutr. 30: 1004–1009 (2001)
Lee SH, Cho HN, Hyun CK, Jew SS. Physiology functional characteristic of silk peptide. Food Sci. Ind. 35: 57–62 (2002)
Sugiyama K, Kushima Y, Muramatsu K. Effect of sulfur containing amino acid and glycine on plasma cholesterol level in rats fed on a high cholesterol diet. Arg. Biol. Chem. Tokyo 49: 3455–3461 (1985)
Kim TM, Ryu JM, Seo IK, Lee KM, Yeon S, Lim W-T, Lee J-Y, Hwang SY, Kim Y-B. Four-week repeated-dose toxicity of silk amino acids in rats. Lab. Anim. Res. 24: 565–573 (2008)
Shin S, Park D, Yeon S, Jeon JH, Kim TK, Joo SS, Lim W-T, Lee JY, Kim Y-B. Stamina-enhancing effects of silk amino acid preparations in mice. Lab. Anim. Res. 25: 127–134 (2009)
Shin S, Yeon S, Park D, Oh J, Kang H, Kim S, Joo SS, Lim W-T, Lee J-Y, Choi K-C, Kim KY, Kim SU, Kim J-C, Kim Y-B. Silk amino acids improve physical stamina and male reproductive function of mice. Biol. Pharm. Bull. 33: 273–278 (2009)
Kang YK, Nam SH, Sohn HO, Lee DW. Inhibitory effects of silkworm-extract (SE) on monoamine oxidase activity in vitro and in vivo. Entomol. Res. 35: 189–193 (2005)
Iancu R, Mohapel P, Brundin P, Paul G. Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson’s disease in mice. Behav. Brain Res. 162: 1–10 (2005)
Zeevalk GD, Manzino L, Sonsalla PK, Bernard LP. Characterization of intracellular elevation of glutathione (GSH) with glutathione monoethyl ester and GSH in brain and neuronal cultures: Relevance to Parkinson’s disease. Exp. Neurol. 203: 512–520 (2007)
Kato N, Sato S, Yamanaka A, Yamada H, Fuwa N, Nomura M. Silk protein, sericin, inhibits lipid peoxidation and tyrosinase activity. Biosci. Biotech. Bioch. 62: 145–147 (1998)
Coombes JS, McNaughton LR. Effects of branched-chain amino acid supplementation on serum creatine kinase and lactate dehydrogenase after prolonged exercise. J. Sport Med. Phys. Fit. 40: 240–246 (2000)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, T.K., Park, D., Yeon, S. et al. Tyrosine-fortified silk amino acids improve physical function of Parkinson’s disease rats. Food Sci Biotechnol 20, 79–84 (2011). https://doi.org/10.1007/s10068-011-0011-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-011-0011-z