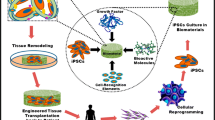
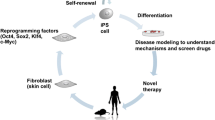
Abstract
The past decade has seen outstanding scientific progress in the field of stem cell (SC) research and clinical application. SCs are convenient both technically and biologically: they are easy to find and to culture and they can differentiate in virtually all tissues and even in whole organs. Induced pluripotent stem cells (iPSs) are a type of pluripotent SC generated in vitro directly from mature cells through the introduction of key transcription factors. The use of iPSs, however tantalizing, poses serious safety concerns because of their genomic instability. Recently, it has been suggested that the main mechanism of SC action relies on paracrine signals. Therefore, the secretome would be primarily responsible for SC effects. The therapeutical use of secretome is safer and more reliable and offers manufacturing, handling and transportation advantages. The authors discuss current applications of SCs with particular respect to bone regeneration stressing the possible risks that may arise from incautious employments of SCs—particularly when associated with stimulating factors. Safety issues hamper the advancement of SC-based innovative therapies and raise the need for novel standards to adequately address and rule out inconsistency and other concerns, considering the permanent nature of SC treatments. Many biological aspects concerning dose, time and site of administration are still to be elucidated. Solid clinical data and trials with long-term follow-ups are highly recommended as a means to evaluate the risk/benefit ratio of each potential intervention and to provide patients with clear and accurate information.


Similar content being viewed by others
References
Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV (1966) Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol 16(3):381–390
Becker AJ, McCulloch EA, Till JE (1963) Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature 197:452–454
Owen M (1980) The origin of bone cells in the postnatal organism. Arthritis Rheum 23(10):1073–1080
Osdoby P, Martini MC, Caplan A (1982) Isolated osteoclasts and their presumed progenitor cells, the monocyte, in culture. J Exp Zool 224(3):331–344
Wang S, Qu X, Zhao RC (2012) Clinical applications of mesenchymal stem cells. J Hematol Oncol 5:19
Mhashilkar AM, Atala A (2012) Advent and maturation of regenerative medicine. Curr Stem Cell Res Ther 7(6):430–445
Mascarenhas S, Avalos B, Ardoin SP (2012) An update on stem cell transplantation in autoimmune rheumatologic disorders. Curr Allergy Asthma Rep 12(6):530–540. doi:10.1007/s11882-012-0298-8
Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringdén O, Developmental Committee of the European Group for Blood and Marrow Transplantation (2008) Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371(9624):1579–1586
Gao Z, Zhang L, Hu J, Sun Y (2013) Mesenchymal stem cells: a potential targeted-delivery vehicle for anti-cancer drug, loaded nanoparticles. Nanomedicine 9(2):174–184. doi:10.1016/j.nano.2012.06.003
Walles T, Giere B, Hofmann M, Schanz J, Hofmann F, Mertsching H, Macchiarini P (2004) Experimental generation of a tissue-engineered functional and vascularized trachea. J Thorac Cardiovasc Surg 128(6):900–906
Atala A (2011) Tissue engineering of human bladder. Br Med Bull 97:81–104
Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK (1999) Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med 5(3):309–313
Tailor J, Andreska T, Kittappa R (2012) From stem cells to dopamine neurons: developmental biology meets neurodegeneration. CNS Neurol Disord Drug Targets 11(7):893–896
Fiorina P, Voltarelli J, Zavazava N (2011) Immunological applications of stem cells in type 1 diabetes. Endocr Rev 32(6):725–754. doi:10.1210/er.2011-0008
Tzouvelekis A, Antoniadis A, Bouros D (2011) Stem cell therapy in pulmonary fibrosis. Curr Opin Pulm Med 17(5):368–373
Maumus M, Guérit D, Toupet K, Jorgensen C, Noël D (2011) Mesenchymal stem cell-based therapies in regenerative medicine: applications in rheumatology. Stem Cell Res Ther 2(2):14. doi:10.1186/scrt55
Baron F, Storb R (2012) Mesenchymal stromal cells: a new tool against graft-versus-host disease? Biol Blood Marrow Transplant 18(6):822–840
Wu C, Dunbar CE (2011) Stem cell gene therapy: the risks of insertional mutagenesis and approaches to minimize genotoxicity. Front Med 5(4):356–371. doi:10.1007/s11684-011-0159-1
Toupadakis CA, Granick JL, Sagy M, Wong A, Ghassemi E, Chung DJ, Borjesson DL, Yellowley CE (2013) Mobilization of endogenous stem cell populations enhances fracture healing in a murine femoral fracture model. Cytotherapy 15(9):1136–1147. doi:10.1016/j.jcyt.2013.05.004
Katagiri W, Osugi M, Kinoshita K, Hibi H (2015) Conditioned medium from mesenchymal stem cells enhances early bone regeneration after maxillary sinus floor elevation in rabbits. Implant Dent 24(6):657–663. doi:10.1097/ID.0000000000000335
Ionescu L, Byrne RN, van Haaften T, Vadivel A, Alphonse RS, Rey-Parra GJ, Weissmann G, Hall A, Eaton F, Thébaud B (2012) Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol 303(11):L967–L977. doi:10.1152/ajplung.00144.2011
Pawitan JA (2014) Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res Int 2014:965849. doi:10.1155/2014/965849
Brooks M (2017) Stem cell research: time for a dose of realism. BMJ 31:356–j443. doi:10.1136/bmj.j443
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676. doi:10.1016/j.cell.2006.07.024
Buganim Y, Faddah DA, Jaenisch R (2013) Mechanisms and models of somatic cell reprogramming. Nat Rev Genet 14(6):427–439. doi:10.1038/nrg3473
Calderaro J, Rebouissou S, de Koning L, Masmoudi A, Hérault A, Dubois T, Maille P, Soyeux P, Sibony M, de la Taille A, Vordos D, Lebret T, Radvanyi F, Allory Y (2014) PI3K/AKT pathway activation in bladder carcinogenesis. Int J Cancer 134(8):1776–1784. doi:10.1002/ijc.28518
Rouhani FJ, Nik-Zainal S, Wuster A, Li Y, Conte N, Koike-Yusa H, Kumasaka N, Vallier L, Yusa K, Bradley A (2016) Mutational history of a human cell lineage from somatic to induced pluripotent stem cells. PLoS Genet 12(4):e1005932. doi:10.1371/journal.pgen.1005932. eCollection 2016i
Koido S, Ito M, Sagawa Y, Okamoto M, Hayashi K, Nagasaki E, Kan S, Komita H, Kamata Y, Homma S (2014) Vaccination with vascular progenitor cells derived from induced pluripotent stem cells elicits antitumor immunity targeting vascular and tumor cells. Cancer Immunol Immunother 63(5):459–468. doi:10.1007/s00262-014-1531-1
Yamashita T, Kawai H, Tian F, Ohta Y, Abe K (2011) Tumorigenic development of induced pluripotent stem cells in ischemic mouse brain. Cell Transplant 20(6):883–891. doi:10.3727/096368910X539092
Bayart E, Cohen-Haguenauer O (2013) Technological overview of iPS induction from human adult somatic cells. Curr Gene Ther 13:73–92
Zhao H, Davies TJ, Ning J, Chang Y, Sachamitr P, Sattler S, Fairchild PJ, Huang FP (2015) A highly optimized protocol for reprogramming cancer cells to pluripotency using nonviral plasmid vectors. Cell Reprogram 17(1):7–18. doi:10.1089/cell.2014.0046
Parekkadan B, Milwid JM (2010) Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng 12:87–117. doi:10.1146/annurev-bioeng-070909-105309
Kunter U, Rong S, Boor P, Eitner F, Müller-Newen G, Djuric Z, van Roeyen CR, Konieczny A, Ostendorf T, Villa L, Milovanceva-Popovska M, Kerjaschki D, Floege J (2007) Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. J Am Soc Nephrol18(6):1754–1764
Iwamoto S, Mihara K, Downing JR, Pui CH, Campana D (2007) Mesenchymal cells regulate the response of acute lymphoblastic leukemia cells to asparaginase. J Clin Invest 117(4):1049–1057
Wend P, Holland JD, Ziebold U, Birchmeier W (2010) Wnt signalling in stem and cancer SCs. Seminars in Cell & Dev Biol 21:855–863
Enders GH (2009) Wnt therapy for bone loss: golden goose or Trojan horse? J Clin Invest 119(4):758–760
Hayflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp Cell Res 25:585–621
Shay JW, Wright WE, Werbin H (1991) Defining the molecular mechanisms of human cell immortalization. Biochim Biophys Acta 1072(1):1–7
Tomasetti C, Vogelstein B (2015) Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 17(6217):78–81
Rubio D, Garcia-Castro J, Martín MC, de la Fuente R, Cigudosa JC, Lloyd AC, Bernad A (2005) Spontaneous human adult SC transformation. Cancer Res 65(8):3035–3039
Wang Y, Huso DL, Harrington J, Kellner J, Jeong DK, Turney J, McNiece IK (2005) Outgrowth of a transformed cell population derived from normal human BM mesenchymal SC culture. Cytotherapy 7(6):509–519
Zhou YF, Bosch-Marce M, Okuyama H, Krishnamachary B, Kimura H, Zhang L, Huso DL, Semenza GL (2006) Spontaneous transformation of cultured mouse bone marrow-derived stromal cells. Cancer Res 66(22):10849–10854
Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S, Xia L, Zhou N, Riddle M, Schroeder TM, Westendorf JJ, McIvor RS, Hogendoorn PC, Szuhai K, Oseth L, Hirsch B, Yant SR, Kay MA, Peister A, Prockop DJ, Fibbe WE, Blazar BR (2007) Sarcoma derived from cultured mesenchymal SCs. Stem Cells 25(2):371–379
Park SI, Soki SN, McCauley LK (2011) Roles of bone marrow cells in skeletal metastases: no longer bystanders. Cancer Microenviron 4(3):237–246
Yamada KM, Cukierman E (2007) Modeling tissue morphogenesis and cancer in 3D. Cell 130(4):601–610
Tasso R, Augello A, Caridà M, Postiglione F, Tibiletti MG, Bernasconi B, Astigiano S, Fais F, Truini M, Cancedda R, Pennesi G (2009) Development of sarcomas in mice implanted with mesenchymal SCs seeded onto bioscaffolds. Carcinogenesis 30(1):150–157
Morris EV, Edwards CM (2016) Bone marrow adipose tissue: a new player in cancer metastasis to bone. Front Endocrinol (Lausanne) 7:90. doi:10.3389/fendo.2016.00090 eCollection 2016
Piccolo S, Cordenonsi M, Dupont S (2013) Molecular pathways: YAP and TAZ take center stage in organ growth and tumorigenesis. Clin Cancer Res 19(18):4925–4930. doi:10.1158/1078-0432.CCR-12-3172
Bell RB, Curtis G (2009) Reconstruction of mandibular continuity defects using recombinant human bone morphogenetic protein 2: a note of caution in an atmosphere of exuberance. J Oral Maxillofac Surg 67:2673–2678
Halme DG, Kessler DA (2006) FDA regulation of stem-cell-based therapies. N Engl J Med 355:1730–1735. doi:10.1056/NEJMhpr063086
Acknowledgements
The authors are grateful to Mr. Peter Rinearson for his assistance in editing the English version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Rights and permissions
About this article
Cite this article
Musacchio, E., Veronese, N. Bone regeneration in the stem cell era: safe play for the patient?. Clin Rheumatol 36, 745–752 (2017). https://doi.org/10.1007/s10067-017-3581-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-017-3581-1