Abstract
Clinically, gout is generally considered as a preferential male disease. However, it definitely does not occur exclusively in males. Our aim was to assess differences in the clinical features of gout arthritis between female and male patients. Five electronic databases were searched to identify relevant original studies published between 1977 and 2007. The included studies had to focus on adult patients with primary gout arthritis and on sex differences in clinical features. Two reviewers independently assessed eligibility and quality of the studies. Out of 355 articles, 14 were selected. Nine fulfilled the quality and score criteria. We identified the following sex differences in the clinical features of gout in women compared to men: the onset of gout occurs at a higher age, more comorbidity with hypertension or renal insufficiency, more often use of diuretics, less likely to drink alcohol, less often podagra but more often involvement of other joints, less frequent recurrent attacks. We found interesting sex differences regarding the clinical features of patients with gout arthritis. To diagnose gout in women, knowledge of these differences is essential, and more research is needed to understand and explain the differences , especially in the general population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gout is a frequent form of arthritis, presenting as a severe and painful inflammatory arthritis, mostly of the first metatarsophalangeal joint (podagra). It occurs suddenly, and in most patients it disappears completely within 5 to 14 days [1].
Gout affects around 1% of adult men in Western countries, mostly aged over 45 years. The estimated incidence of gout in these countries is 0.6 to 2.1 per 1,000 per year, with a prevalence of 3 to 7.5 per 1,000 per year [2–4]. Accumulating data support an increase in the prevalence of gout that is potentially attributable to recent shifts in diet, lifestyle, medical care, and increased longevity. The increased longevity of the population in industrialized countries may contribute to a higher prevalence of gout through the disorder’s association with age-related diseases such as hypertension and cardiovascular diseases. The increasing prevalence of gout worldwide indicates a need for improved effort to identify these patients early in the disease process [5].
Clinically, gout is often considered a preferential male disease. The condition is more common in men than in women. In women, it mainly becomes manifest in the postmenopausal period. Hippocrates was the first who stated that it was a sex-related disease. Among patients younger than 65 years, men have a fourfold greater prevalence than women [6]. However, the incidence of gout in the elderly has a more equal sex distribution. The impact of female hormones cannot be neglected because estradiol can lower serum urate in females, and the serum urate rises after the menopause [7]. In patients older than 65 years, the sex gap narrows to one woman to every three men with gout and/or hyperuricemia [5]. Research about gout arthritis in the general population is rare; even less is known about gout in female patients in general population. The aim of this review is to evaluate the available studies on the sex differences in clinical features of gout arthritis.
Therefore, our research question was: What is known in medical literature about the clinical features of gout in females compared to males?
Methods
The present review was conducted in cooperation with a trained librarian (EP), according to the methodology of the Effective Practice and Organization of Care module from the Cochrane library [8]. Electronic searches were undertaken in MEDLINE, EMBASE, the Cochrane Clinical Trials Register, and Web of Science. The search strategy aimed to identify original relevant research papers published between 1977 and 2007 on gout and sex differences. It consisted of the AND combination of two main concepts: gout and the gender-specific filter . Meaning: Gout AND sex characteristics OR sex distribution OR sex factors OR sex differences OR gender identity OR gender OR sex distribution OR women’s health OR menopause OR pregnancy OR breast feeding OR menstruation OR gonadal hormones OR men’s health—in Mesh headings and text words [9].
The strategy used to identify relevant articles was adapted to the specific search criteria required for each database. Hyperuricemia can be present in a patient who does not have acute gout arthritis, and a patient, however seldom, can have gout without hyperuricemia. For this reason, we left the term hyperuricemia out of the search strategy. No age limit was used. Language was limited to Dutch, English, German, and French. We started the selection in 1977 because we used the Wallace gout criteria defined in 1977, later called the American College of Rheumatology (ACR) criteria of the American Rheumatology Association [10]. Additional studies were identified by searching the reference lists of relevant trials and reviews. Details of the database searches can be obtained from the author.
Selection
To select studies for further assessment, two observers (KD and TT) have independently reviewed the titles, abstracts, and keywords of every record retrieved. Articles were selected if the information from title, abstract, or keywords included gout not caused by another underlying disease and if the article reported an explicit comparison of male and female clinical features and a clear description about how they made the diagnosis of gout. If the information of title, abstract section, or keywords was unclear, the full article was retrieved for clarification. If no consensus was reached about whether to include a study, a third observer (ALJ) was asked to make a definite decision. Interobserver agreement for study selection was measured using the kappa statistic.
The two reviewers assessed the quality of each retrieved trial independently, using the “Quality criteria list on cohort studies” (CBO 2003: http://www.cbo.nl/product/richtlijnen/handleiding_ebro/article20050427141202/articleCBObasic_view). In this way, we checked whether the study met the international guideline criteria. We checked the full description of the stated aim of the study, study population, patient characteristics such as ethnicity, socioeconomic status, and setting (inpatient or outpatient, general practice), definition of the study inclusion and exclusion criteria, observation period, outcome measurements, statistic analysis, and data presentation. If any of this information was missing, the article was excluded.
Quality assessment of selected articles
The included full articles were graded and given a quality score based on the following information: method of diagnosing gout, female versus male comparisons, the absolute number and the age of the female patients, and the type of population and adequate outcome measures (Table 1). The diagnosis of gout was considered accurate if there was documentation on urate crystals aspirated from an affected joint or, failing this, if the clinical characteristics recorded were sufficient to fulfill the ACR diagnostic criteria of gout [10]. We also included studies in which the diagnosed gout was based on a dramatic clinical improvement of the arthritis as response to a treatment with colchicine.
Results
From MEDLINE, we retrieved 178 original articles. The two observers (KD, TT) selected 69 articles on title and abstract (kappa 0.57). From EMBASE and the Cochrane Library, we retrieved another 20 articles. In the Web of Science, we searched for articles quoted by the authors of our top ten articles. No new articles were found. Out of the 89 full articles, we selected 14 original studies with specific information on sex differences of gout and a clear description about how they made the diagnosis of gout. These articles were scored according to the quality criteria mentioned in Table 1. The maximum score was 5. The minimum score for inclusion was set on 2.5. Nine articles were included, and five were excluded (Table 2) [11, 15].
Two of the nine articles were conducted in a general population and seven in a hospital. The age of the male patients ranged from 53 to 70 years and of the female patients from 49 to 61 years. The ethnicity of the study population differed in the studies. In two studies [16, 17], the diagnosis of gout was made by synovial tap, in one by using both synovial tap and the ACR criteria [18], in four by the ACR criteria [19–22], and in two by ACR criteria or clinical improvement on treatment with colchicines [23]. The best way to classify a patient as having acute gout is to demonstrate characteristic sodium urate monohydrate crystals in the joint fluid. If the diagnosis is based on colchicine of ACR criteria, other causes of arthritis were also included in the studies. Because of this, we reviewed the studies with crystal-proven gout separately from the studies using the ACR criteria and the colchicine definition.
The diagnosis of gout based on synovial fluid analysis
Two studies are based on a crystal-proven gout [16, 17]. In the study of Puig et al., in 89% of the study population, the gout was crystal-proven (n = 40) [18]. Because of the high percentage, we include this study also in the group “crystal-proven gout” (Table 3).
Patient characteristics
The age of onset of gout as described in the three studies was on average 8.1 years later in women, and the duration of the gout at study entry was significantly higher in men. Gallerani et al. only studied the influence of the season on gout attacks and showed a significant peak of gout attacks in April in male patients, 36% of all the attacks [17]. Most of the women were postmenopausal at the onset of gout (86% and 92%).
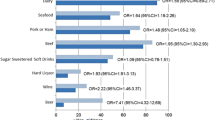
Considering the use of diuretics, female gout patients received diuretics significantly more often than male patients, 57% of the women vs 14% of the men [18] and 83% of the women vs 47% of the men [16]. In the same studies, men with gout were more likely to drink alcohol than women, 14% of the women vs 55% of the men [18] and 10% of the women vs 45% of the men [16]. Puig et al. also analyzed sex differences in obesity in gout patients and found no differences between men and women.
Joint location
Puig et al. and Lally et al also described the location of the arthritis [16, 18]. Men seemed to have a higher prevalence of podagra at their first attack, 52% of the women vs 57% of the men [16]. Puig et al. found significantly more tophi in female gout patients compared to male, 27% in women vs 10% in men [18]. Lally et al. found a higher prevalence of polyarticular gout in male patients, 56% of the women vs 80% of the men [16]. They found no sex differences in tophaceous gout, but in their study the upper limb seemed to be more involved in male patients, 44% in women vs 47% in men [16].
Comorbidities
Finally, Puig et al. and Lally et al. reported the incidence of “gout-associated comorbidities” as diabetes, hypertension, and renal insufficiency [16, 18]. In both studies, an association was found between renal insufficiency and postmenopausal women, 54% of the women vs 11% of the men [18] and 30% of the women vs 12% in men [16]. Puig et al. considered that hypertension was more common in female than in male gout patients, 78% of the women vs 14% of the men [18]. The sex distribution on diabetes and gout was equal [18].
Gout according to the ACR criteria or clinical improvement on colchicine
Six studies did not base the diagnosis on a crystal-proven gout (Table 4).
Patient characteristics
The age of onset of gout was on average 9.2 years later in women (mean age 66 years) compared to men (mean age 54 years), and the duration of the gout at study entry was significantly higher in men. Most of the women were postmenopausal at the onset of the gout (66–95%). Three studies described the use of diuretics [19–21]. In two studies, female gout patients received diuretics significantly more often than male patients, 77% in women vs 40% in men [19] and 72% in women vs 48% in men [20]. Three studies reported the use of alcohol in gout patients [20–22]. They found that men with gout were more likely to drink alcohol than women, 2% of the women vs 20% of the men [20], 7% of the women vs 16% of the men [21], and 55% of the women vs 82% of the men. Tikley et al. found sex differences in the relation between obesity and gout. In women, they did not found any relation between the body mass index (BMI) and gout, but they found a higher prevalence of gout in men with a BMI > 25 (odds ratio 7.8) [22]. Harrold et al. studied the recurrence of gout attacks and reported a significantly higher number in men [19]. Gout in the family history was equally distributed between the sexes [21].
Joint location
The studies of De Souza et al. and Deesomchok et al. described the location of the arthritis [21, 23]. Men had a higher prevalence of podagra at their first attack, 23% of the women vs 45% of the men [23]. During the gout disease period, the sex differences in the location of the recurrent attack widened, with an increasingly higher prevalence of podagra in men [21]. Female patient more often had other joints involved, such as ankle, fingers, and upper limb [23]. Polyarticular gout seemed to be more related to female gout patients, 63% of the women vs 39% of the men [20] and 41% of the women vs 24% of the men [23]. The presence of tophi differed in the various studies, 39% of the women vs 26% of the men [20], 30% of the women vs 48% of the men [21], 18% of the women vs 31% of the men [24], and 34% of the women vs 18% of the men [23].
Comorbidities
Five studies reported the incidence of several “gout-associated comorbidities” [19–23]. In four of them, hypertension was more common in female than in male gout patients, 81% of the women vs 57% of the men [19], 81% of the women vs 77% of the men [21], 45% of the women vs 39% of the men [23], and 65% of the women vs 59% of the men [22]. In two studies, this difference was significant [19, 22]. Five studies considered diabetes [19–23]. In one study, diabetes was significantly more prevalent among female than among male gout patients, 30% of the women vs 17% of the men [19]. The other four were contradicting.
Four studies analyzed the relationship between gout and cardiovascular heart disease. These results were also inconsistent, 25% of the women vs 19% of the men [19], 26% of the women vs 57% of the men [20], 25% of the women vs 16% of the men [21], and 0% of the women vs 11% of the men [23]. Dyslipemia was more common in women (42% vs 38%) [18–21] and Deesomochok et al. did not find a sex difference in cerebral vascular accident in gout patients [23]. In this study, a significantly higher prevalence of hematologic malignancies was found in female gout patients, 22% of the women compared to 3% of the men [23].
Renal insufficiency was studied in five studies, and in all an association was found with gouty arthritis in postmenopausal women (in three of them, the association was significant), especially in those with preexisting joint disease, 18% of the women vs 10% of the men [19], 25% of the women vs 15% of the men [20], and 22% of the women vs 12% of the men [24].
We studied the conclusions of the five excluded studies and compared these with the nine included papers. No significant difference in outcome variables was found between the two groups.
Discussion
We selected nine articles which varied largely in the characteristics of the study population such as a hospital population, outpatient department or general population, the number of postmenopausal women, age, and the ratio of women and men. Moreover, the method of classifying gout varied from using the ACR criteria to detecting urate crystals. These factors influence the clinical features and make it difficult to compare the results. Therefore, we divided the studies in one group diagnosed on a synovial fluid analysis and another group diagnosed on the ACR/colchicine criteria. Nevertheless, there are great similarities in results in both groups. Our conclusions are that women are almost a decade older at the onset of gout arthritis, have more associated comorbidities such as hypertension and renal insufficiency, and use less alcohol. Also, the typical location in the first toe is less frequent in women, and women are more likely to be taking diuretics. In the crystal-proven gout group, polyarticular gout tends to be more prevalent in male while in the ACR group polyarthritis seems to be related to women.
The difference in age between men and women at the onset of gout is remarkable. One of the reasons is possibly the menopause. After the menopause, the incidence of gout is high compared to the reproductive age [16, 18, 23, 24] Kim et al. suggested a possible role of 17-beta-estradiol in the regulation of purine biosynthesis and uric acid metabolism and lowered serum urate [7]. Also, Hak et al. found in a recent large prospective study that menopause increases the risk of gout [25].
Women with gout have more often renal insufficiency and hypertension, and they more often use diuretics. Is it because of the older age of female gout patients that they had a higher percentage of renal dysfunction? Lally et al. and Bero et al. controlled their study results for age at the gout onset, and after this correction they still found a higher prevalence of renal insufficiency and diuretic use in female gout patients [4, 8, 16]. Renal dysfunction reduces urate excretion, and hyperuricemia has been found to increase tubular reabsorption of sodium and may thus be predisposing for hypertension and diuretic use. Conversely, hypertension can induce renal dysfunction [26]. Women in general use diuretics much more frequently than men: a causal relationship between gout and diuretics is therefore unclear.
Alcohol increased the risk of gout but plays a less important role in the development of gout in women. Alcohol causes hyperuricemia by reducing renal excretion of urate as a result of increased adine nucleotide turnover.
Female gout patients differ in the location of the gout arthritis. In women, not only podagra is involved but also other joints such as fingers and ankle. Therefore, gout should be considered seriously in the differential diagnosis of elderly women with an acute (oligo)arthritis, especially of the ankle. These atypical locations may cause a delay in the diagnosis because of unfamiliarity of physicians with the atypical presentation of gout in women. Especially since a punction of monosodium urate crystals in joint fluid is seldom used in primary care in the diagnostic procedure.
Another reason for a delay in the diagnosis in women may be the severity of coexisting diseases because of their older age compared to men, which overshadows gout. Studies in which the arthritis location is described in detail are rare. It seems that gout in female patients has fewer recurrences.
Harrold et al. performed the first population-based study with a high number of female patients [19]. In this study, female gout was more associated with hypertension, dyslipemia, chronic heart disease, peripheral arterial disease, diabetes, and renal insufficiency compared to male patients with gout. The other studies examining sex differences in clinical characteristics of gout were very small studies, and only the study of Chang et al. was population-based [24].
Strengths and limitations of this study
This is, as far as we know, the first systematic review about clinical features of women with gout arthritis.
This study has some limitations. Firstly, most studies are based on physician diagnosis by using the ACR criteria or the response to colchicine, a definition that is subject to misclassification. The unreliable diagnosis of gout is a problem. If we had strictly accepted the gout diagnosis on the basis of urate crystals, only two studies would have been included [16, 17]. Malik et al. found a positive predictive value of 66% for ACR criteria compared to the golden standard crystal identification by synovial tap [27]. So, up to a third of the cases included in these studies might have false positives. Despite this problem, the results in the crystal-proven gout studies were quite equal to the studies using the ACR criteria, and in daily practice the diagnosis of gouty arthritis is based on clinical grounds without the use of crystal identification in de synovial fluid.
Another problem is the small number of women in most studies. Only the study of Harrold et al. performed a population-based study with a high number of female patients [19]. In all other included studies, the total number of included patients, especially women, is much too low to explore sex differences in gout-related comorbidities. Besides, most studies are performed in a clinical setting and depend therefore on presented morbidity. Only the studies of Harrold et al. and Chang et al. were population-based [15, 18].
Conclusion
This review is a unique study and a start for further research, in order to realize evidence-based diagnostic procedures and treatments for female patients with gout arthritis in general population.
Our systematic review about sex differences in gout arthritis draws attention to some of the less-known characteristics of gout. Conclusions must be drawn with great caution because of the small number of available studies on female gout, which however were carried out in different populations, with different criteria and mostly small numbers of women.
We found differences in the clinical features of gout arthritis between men and women. The onset of gout occurs at a later age in women; they are more likely to have comorbidities such as hypertension or renal insufficiency, and they use diuretics more often. Men with gout are more likely to drink alcohol, and they have a higher prevalence of typical podagra than female patients. Women may more often have other joints involved than just one toe, and the gout recurs less often.
More research in general population is necessary to identify sex differences in clinical features, in order to avoid a possible delay in diagnosing gout and recognize patients who are at risk for developing gout. We need population-based studies with a large number of female patients. In new research, more attention also have to be given to the difference in first recurrent episodes of gout. Do clinical features differ in these two groups?
Because gout is a rare disease in women and it can have an unusual way of manifesting itself, it is very important to recognize the symptoms. Physicians should consider gout arthritis especially in elderly female patients with hypertension, diuretic use, and renal insufficiency and arthritis in one or more joints. It is easy to overlook or pay little attention to the chronic joint problems because the female patients are older and frequently have chronic diseases. The recognition of gout even with an atypical course is important because there are therapeutic strategies to reduce recurring unnecessary pain and complications like tophi deformities, nephropathy, and joint destruction.
References
Bieber JD, Terkeltaub RA (2004) Gout: on the brink of novel therapeutic options for an ancient disease. Arthritis Rheum 50(8):2400–2414
Arromdee E, Michet CJ, Crowson CS, O'Fallon WM, Gabriel SE (2002) Epidemiology of gout: is the incidence rising? J Rheumatol 29(11):2403–2406
Janssens HJ, van de Lisdonk EH, Bor H, van den Hoogen HJ, Janssen M (2003) Gout, just a nasty event or a cardiovascular signal? A study from primary care. Fam Pract 20(4):413–416
Janssens HJ, van de Lisdonk EH, Janssen M, van den Hoogen HJ, Verbeek AL (2006) Gout, not induced by diuretics? A case–control study from primary care. Ann Rheum Dis 65(8):1080–1083
Saag KG, Choi H (2006) Epidemiology, risk factors, and lifestyle modifications for gout. Arthritis Res Ther 8(Suppl 1):S2
Wallace KL, Riedel AA, Joseph-Ridge N, Wortmann R (2004) Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol 31(8):1582–1587
Kim KY, Schumacher HR, Hunsche E, Wertheimer A, Kong S (2003) A literature review of epidemiology and treatment in acute gout. Clin Ther 25:1593–1617
Bero LA, Grilli R, Grimshaw JM, Hervey E, Oxman AD, Thomson MA (1998) Closing the gap between research and practice: an overview of systematic reviews of interventions to promote the implementation of research findings. The Cochrane effective practice and organization of care review group. BMJ 317(7156):465–468
Keuken DG, Haafkens JA, Moerman CJ, Klazinga NS, ter Riet G (2007) Attention to sex-related factors in the development of clinical practice guidelines. J Womens Health 16(1):82–92
Wallace SL, Robinsom H, Masi AT, Decker JL, McCarty DL, Yu FT (1977) Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum 20(3):895–900
Currie WJ (1978) The gout patient in general practice. Rheumatol Rehabil 17(4):205–217
ter Borg EJ, Rasker JJ (1987) Gout in the elderly, a separate entity? Ann Rheum Dis 46(1):72–76
Cassim B, Mody GM, Deenadayalu VK, Hammond MG (1994) Gout in black South Africans: a clinical and genetic study. Ann Rheum Dis 53(11):59–62
Kowalec JK, Krey PR (1978) Gout in an urban setting. J Med Soc N J 75(9):617–620
Macfarlane DG, Dieppe PA (1985) Diuretic-induced gout in elderly women. Br J Rheumatol 24(2):155–157
Lally EV, Ho G, Kaplan SR (1986) The clinical spectrum of gouty arthritis in women. Arch Intern Med 146(11):2221–2225
Gallerani M, Govoni M, Mucinelli M, Bigoni M, Trotta F, Manfredinil R (1999) Seasonal variation in the onset of acute microcrystalline arthritis. Rheumatology (Oxford) 38(10):1003–1006
Puig JG, Michan AD, Jimenez ML, Perez de Ayala C, Mateos FA, Capitan CF et al (1991) Female gout. Clinical spectrum and uric acid metabolism*2ws KT in. Arch Intern Med 51(4):726–732
Harrold LR, Yood RA, Mikuls TR, Andrade SE, Davis J, Fuller J et al (2006) Sex differences in gout epidemiology, evaluation and treatment. Ann Rheum Dis 65:1368–1372
Meyers OL, Monteagudo FS (1986) A comparison of gout in men and women*2. A 10-year experience. S Afr Med J 70(12):721–723
De Souza AW, Fernandes V, Ferrari AJ (2005) Female gout: clinical and laboratory features. J Rheumatol 32(11):2186–2188
Tikly M et al (1998) Risk factors for gout: a hospital-based study in urban black South Africans. Rev Rhum Engl Ed 65(4):225–231
Deesomchok U, Tumrasvin T (1989) A clinical comparison of females and males with gouty arthritis. J Med Assoc Thai 72(9):510–515
Chang SJ, Chen CJ, Hung HP, Ou TT, Ko YC (2004) Community-based study in Taiwan aborigines concerning renal dysfunction in gout patients. Scand J Rheumatol 33(4):233–238
Hak AE, Curhan G, Grodstein FD, Choi HK (2009) Menopause, postmenopausal hormone use and risk of incident gout. Ann Rheum Dis. doi:ard.2009.109884v1
Cappuccio FP, Stazzullo P, Farinaro E, Trevisan M (1993) Uric acid metabolism and tubular sodium handling: results from a population based study. JAMA 270:354–359
Malik A, Schumacher R, Dinnella JE, Clayburne GM (2009) Clinical diagnostic criteria for gout. Comparison with the gold standard of synovial fluid crystal analysis. J Clin Rheumalog 15:22–24
Acknowledgement
Elmie Peters, Librarian UMCN, St. Radboud.
Disclosures
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Dirken-Heukensfeldt, K.J., Teunissen, T., van de Lisdonk, E. et al. “Clinical features of women with gout arthritis.” A systematic review. Clin Rheumatol 29, 575–582 (2010). https://doi.org/10.1007/s10067-009-1362-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-009-1362-1