Abstract
Expression of the ALK gene strongly correlates with the WNT-activated medulloblastomas, which are routinely identified by detection of CTNNB1 mutation. However, some tumors have mutations in other than CTNNB1 genes. Therefore, we investigated if ALK expression may identify WNT-activated tumors without CTNNB1 mutation. In addition, we examined if ALK expression may differentiate WNT-activated medulloblastoma from other malignant posterior fossa tumors. ALK expression was analyzed using immunohistochemistry (clone D5F3) in 70 patients with posterior fossa tumours. Among 55 medulloblastomas, 6 tumors showed ALK expression in > 50% of tumor cells. In one tumor, with ALK positive reaction, negative nuclear reaction against β-catenin and the lack of CTNNB1 mutation, next generation sequencing revealed a presence of pathogenic variant c.3366_3369del in the APC gene, with homozygous deletion leading to inactivation of both copies in tumor cells. MLPA analysis displayed the presence of chromosome 6 monosomy, therefore, confirming the WNT type of this tumor. All analyzed 19 anaplastic ependymomas, 4 choroid plexus carcinomas and 2 atypical teratoid rhabdoid tumors were immunonegative for ALK expression. Therefore, we propose, that immunohistochemical detection of ALK protein should be highly recommended in routine investigation, in parallel to already established methods for identification and differentiation of WNT-activated medulloblastoma.
Similar content being viewed by others
Introduction
Medulloblastoma is the most common malignant pediatric brain tumor. Molecular studies revealed the existence of several subgroups of this disease which depend on distinctive profiles of genes expression and DNA alterations [1,2,3]. The four genetically defined principal subtypes: Wingless (WNT), Sonic Hedgehog (SHH), Group 3 and Group 4 medulloblastoma are now recognized as distinct biological entities and are acknowledged in the updated World Health Organization (WHO) 2016 classification of tumors of the central nervous system [4, 5].
Importantly, several independent studies confirmed correlations between the molecular groups and clinical features, including survival of patients [2, 6]. Particularly, patients with the WNT-activated tumors have a favorable outcome of disease. In consequence, the recent SIOP-Europe trial (PNET 5-MB) introduces a reduction of the radiation dose for those patients, thus potentially reducing the severe side effects of the current treatment. Therefore, identification of patients with the WNT-activated tumors is essential because it results in clinical consequences.
Currently, as indicated in the 2016 WHO classification, there are several approaches for the identification of WNT-activated tumors, including detection of CTNNB1 mutation, immunohistochemical positive nuclear reaction against β-catenin and the presence of chromosome 6 monosomy. Nevertheless, some tumors may not be recognized by such approach due to e.g. up-regulation of the WNT pathway by presence of mutations in other than CTNNB1 genes, namely APC, AXIN 1 or AXIN 2 [7,8,9]. In addition, multigene expression or methylation profiling may be applied for sub-grouping purposes, but these techniques are difficult to introduce in everyday diagnostic hospital practice.
We have recently described an association between ALK and WNT-activated tumors. Presence of inherited ALK variant p.M1199L was identified in this type of disease [10] but more importantly, ALK expression, both at the RNA and protein levels, was strongly associated with the WNT-activated type of tumors [11].
Therefore, we further investigated if expression of ALK at the protein level using immunohistochemistry alone may detect WNT-activated tumors, especially those with negative nuclear reaction against β-catenin. In addition, we examined if the ALK protein expression may differentiate WNT-activated medulloblastomas from other histologically misleading high grade malignancies located in the posterior fossa in pediatric cases.
Materials and methods
Patients and tumor material
Overall, 70 patients with infratentorial high grade tumors diagnosed in The Children’s Memorial Health Institute (CMHI) in Warsaw, Poland, were included in the analysis. 55 patients were diagnosed with medulloblastoma (WHO grade IV), 19 patients with anaplastic ependymoma (WHO grade III), 4 patients with choroid plexus carcinoma (CPC, WHO grade IV) and 2 patients with atypical teratoid rhabdoid tumors (AT/RT, WHO grade IV). All patients were treated according to the protocol of the Polish Pediatric Neurooncology Group (PPNG).
Informed consent was obtained to use tumor material according to the procedures outlined by the CMHI’s Ethical Committee.
Analysis was performed on formalin-fixed paraffin embedded (FFPE) tissue samples collected at diagnosis. All tumors were retrospectively reviewed according to a recent WHO 2016 criteria [4, 5].
Detection of molecular subtypes of medulloblastomas
Molecular subtypes of medulloblastomas were detected, as recommended by the 2016 WHO classification:
-
1.
WNT-activated tumors were identified by the presence of at least two features: CTNNB1 mutation, immunohistochemical positive nuclear reaction against β-catenin (#760-4242, clone 14, Cell Margue, dilution 1.68 µg/ml) and the presence of chromosome 6 monosomy detected by multiplex ligation-dependent probe amplification (MLPA).
-
2.
SHH-activated tumors were determined by the presence of immunohistochemical positive reaction with anti-GAB1 (Abcam, Cambridge, USA, #ab27439 and/or ab #59362, dilution 1:100) and anti-YAP1 (Santa Cruz Biotechnology, Dallas, USA, #sc-101199, dilution 1:50) antibodies, as described by Ellison et al. [12].
-
3.
The remaining tumors that were negative for the above features were assigned as non-WNT, non-SHH tumors.
Procedures for the detection of mutations in exon 3 of CTNNB1 and monosomy of chromosome 6 by MLPA are described elsewhere [13].
Detection of ALK expression by immunohistochemistry
Expression of ALK protein and scoring were described previously [11]. Briefly, antibody clone D5F3 #3633 (Cell Signaling, Denver, MA, USA) was used at dilution 1:250 and antigen retrieval was performed using EnVision FLEX HIGH pH solution (DAKO) for 30 min. at 99 °C. ALK scoring was applied as the following: 0 (negative) for 0–10%, 1 + for > 10–50%, 2 + for > 50–100% of positive tumor cells. Whole preparations were scanned in Hamamatsu NanoZoomer 2.0 RS scanner at original magnification 40 ×.
Detection of molecular defects in patient without CTNNB1 mutation and positive ALK reaction using next-generation and Sanger sequencing
Next-generation sequencing (NGS) using genomic DNA extracted from patient’s leucocytes and tumor tissue was performed on a HiSeq 1500 using TruSight One Sequencing Panel (Illumina) according to the manufacturer’s instructions. Generated reads were aligned to the hg19 reference human genome. The detected variants were annotated using Annovar [14]. Bioinformatics analysis was performed as previously described [15]. Alignments were viewed with Integrative Genomics Viewer v.2.2.79 [16]. The nomenclature of molecular variants follows the Human Genome Variation Society guidelines (HGVS, http://www.hgvs.org/mutnomen) and referral to the cDNA sequences follows the Human Gene Mutation Database (HGMD, http://www.hgmd.af.au.uk).
The presence of the APC variant identified by NGS was analyzed by Sanger sequencing. The PCR reactions were carried out with the following primers: APC_16F: TTTGGACAGCAGGAATGTG and APC_16R:GGTCTCTCTTCTTCTTCATGCTG. Bidirectional sequencing was performed using a 3130 genetic analyser (Applied Biosystems, Foster City, CA, USA). The sequences were determined on both DNA strands from at least two independent PCR products. The analyzed sequence fragments were compared with the APC cDNA (Gen-Bank RefSeq:NM_000038.5) and protein (GenBank RefSeq:NP_000029.2) sequences using Mutation Surveyor software version 3.30 (Soft Genetics, LLC, State Collage, PA, USA). Variant positions were numbered according to HGVS recommendations (with + 1 corresponding to the A of the ATG translation initiation codon in the appropriate reference sequence).
Results
Patients with medulloblastoma and tumors histology
The average age of 55 medulloblastoma patients at diagnosis was 8 years, range 1–16 years. 34 patients were males, 21 patients were females. The histological diagnosis of medulloblastoma was classic in 40 cases, large cell/anaplastic (LCA) in 12 cases, desmoplastic/nodular (DN) in 2 cases, medulloblastoma with extensive nodularity (MBEN) in one case.
Detection of ALK expression by immunohistochemistry in medulloblastoma
Overall 55 medulloblastomas were analyzed by immunohistochemistry for ALK expression. 6 tumors showed positive reaction with score 2 + (> 50–100% of positive tumor cells) and one tumors with score 1 + (> 10–50% of positive tumor cells). The remaining 48 tumors showed negative reaction (score 0, 0–10% positive tumor cells).
Among six tumors with ALK score 2 + positive reaction, four tumors showed also positive nuclear reaction against β-catenin and two tumors showed only cytoplasmic immunostaining. No CTNNB1 mutation was detected in both cases with cytoplasmic reaction only, however, both tumors showed the presence of monosomy 6 in MLPA analysis.
Therefore, ALK positive tumors with score 2 + belonged either to the WNT group (4 tumors) or could not be classified (2 tumors). They were all of classic variant, four patients were female and two patients were male, with age from 3 to 16 years at diagnosis. Two tumors were located in cerebello-pontine angle (CPA) and four tumors had cerebellar midline location.
One tumor with positive score 1 + was classified as non-WNT/non SHH tumor and all 48 negative tumors belonged to either the non-WNT/non-SHH group (44 tumors) or SHH group (4 tumors).
Analysis of ALK immunopositive tumor without CTNNB1 mutation
Two ALK positive tumors with strong 2 + reaction, negative immunohistochemical nuclear reaction against β-catenin and lack of CTNNB1 mutation could not be classified as the WNT-activated tumors according to the criteria described in the methods section. However, the presence of monosomy 6 strongly suggests that they belong to the WNT group, although the mechanism of the activation of the WNT pathway is unknown. Subsequently, we performed NGS analysis in one case, where frozen tumor and blood tissues were available, to identify possible presence of other than CTNNB mutations. DNA analysis revealed a presence of pathogenic variant c.3366_3369del, in APC gene (RefSeq RefSeq:NM_000038.5), which creates a frame shift starting at codon Asn1122. The new reading frame ends in a stop codon 3 positions downstream, p.(Asn1122Lysfs*3). Detected variant was reported as a disease causing mutation by Human Gene Mutation Database (CD920811). In silico prediction by various algorithms confirmed the pathogenic impact of identified deletion on the encoded protein. Detected variant was present in heterozygous state in patient’s leukocytes in contrast to tumor DNA where homozygous deletion leading to inactivation of both copies (loss of heterozygosity) of APC gene was observed (Fig. 1).
Medulloblastoma tumor with APC mutation. Magnetic resonance image of the tumor with cerebellar midline location (a); ALK immunopositive reaction present in > 80% of tumor cells (b); result of NGS analysis displaying the presence of the c.3366_3369del APC variant in heterozygous state in DNA extracted from patient’s leukocytes (c) and in homozygous state in tumor sample (d). Preparation was scanned at original magnification × 40 and digital magnification presented on image is × 20
The presence of the APC variant identified by NGS was confirmed by Sanger sequencing. As well as CTNNB1 mutation, APC mutation is also involved in up-regulation of the WNT pathway. Therefore, tumor with the presence of APC mutation and monosomy of chromosome 6 can be classified as the WNT type of medulloblastoma.
The patient was 12 years old male, with tumor of classic histology and cerebellar midline location, without metastases and alive 3 years since diagnosis. In addition, he suffered from polyposis coli condition, what is in line with the molecular findings.
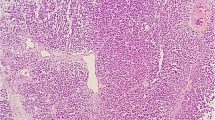
Detection of ALK expression by immunohistochemistry in anaplastic ependymoma, CPC and AT/RT located in the posterior fossa
All 19 anaplastic ependymomas, 4 CPC and 2 AT/RT were negative for ALK expression. Several ependymomas were located in CPA region, similar to two cases of analyzed medulloblastomas. However, they showed dissimilar ALK immunoreactivity. Examples of ALK immunopositive medulloblastoma and ALK immunonegative ependymomas located in the CPA are presented in Fig. 2.
Discussion
As we showed previously, a strong correlation exists between the presence of ALK expression and the WNT type of medulloblastoma, both at the RNA and protein levels. Interestingly, in one tumor with the presence of ALK protein expression, neither mutation in exon 3 of the CTNNB1 gene nor nuclear β-catenin immunoreactivity were detected, but the tumor was classified to the WNT group by application of NanoString method [11]. This raises the possibility that currently recommended methods for routine identification of WNT-activated tumors may be not sufficient, without an application of multigene expression or methylation profiling.
Following this, in the present study, we extended analysis on additional 55 medulloblastomas, with a focus on ALK positive cases. Again, we detected two tumors with positive ALK immunoreactivity but without mutation in CTNNB1 gene and negative nuclear reaction for β-catenin. NGS analysis of both tumor and blood samples in one patient revealed the presence of mutation in the APC gene, with homozygous deletion in tumor cells. In addition, MLPA analysis showed the presence of chromosome 6 monosomy, therefore, reassuring that this tumor belonged to the WNT group.
Mutations in the APC gene in medulloblastoma have been known for some time [7] and very recently, germline mutations were identified in a large series of patients with medulloblastoma using NGS analysis [17]. APC gene is one of the consensus medulloblastoma predisposition genes and patients develop specifically WNT-activated type of tumors. APC mutations were the most frequent (71%) mutations in WNT-activated tumors where the CTNNB1 gene was not altered.
It is, therefore, possible that WNT-activated tumors can be more frequent than already detected by routine diagnostic methods. This may be related to the fact that nuclear β-catenin immunoreactivity is sometimes only focal or absent even in tumors harboring CTNNB1 mutations [12] and other mutations activating the WNT pathway are not routinely investigated. Such cases may be classified as non-WNT/non-SHH tumors, with clinical consequences for the patient and potential impact on survival analyses. Therefore, the fact that ALK expression is positive in APC mutated tumor reinforces the usefulness of this marker for detection of the WNT type of medulloblastoma.
WNT-activated tumors may be located in cerebellar midline or the CPA region, as opposite to SHH tumors located usually in cerebellar hemispheres and non-WNT/non-SHH tumors characterized by cerebellar midline location.
In pediatric patients, the differential diagnosis for medulloblastomas includes other primary malignant brain tumors that arise in the posterior fossa. The most challenging malignances to be considered are: AT/RTs, CPCs and anaplastic ependymomas. These highly cellular neoplasms can show the advantage of small round poorly differentiated elements, similar to medulloblastoma. For instance, anaplastic ependymoma, located in CPA or cerebellar midline regions, may be confused with medulloblastoma, especially when the later tumor shows the presence of pseudorosette-like structures. In addition, AT/RT often includes a small blue cells population, in addition to characteristic rhabdoid phenotype, thus mimicking the large cell/anaplastic medulloblastoma. Similarly, CPC may contain variable population of poorly differentiated cells that can often be difficult to recognize solely on the basis of histology. Therefore, the identification of these lesions requires application of a wide panel of antibodies or molecular studies.
Based on our results, we indicate that ALK protein expression may easily differentiate WNT-activated medulloblastomas from other histologically similar high-grade pediatric tumors located in the posterior fossa (cerebellum/fourth ventricle/ cerebellopontine angle). Nevertheless, more cases should be examined for differentiation purposes to confirm our findings.
In summary, we propose that the immunohistochemical detection of ALK protein should be routinely investigated, in parallel to already established methods for identification of WNT-activated medulloblastomas. In case of positive ALK reaction and absence of CTNNB1 mutation, other genes, especially APC, should be analyzed.
References
Kool M, Koster J, Bunt J, Hasselt NE, Lakeman A, van Sluis P et al (2008) Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One 3(8):e3088. https://doi.org/10.1371/journal.pone.0003088
Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S et al (2011) Medulloblastoma comprises four distinct molecular variants. J Clin Oncol 29:1408–1414
Cavalli FMG, Remke M, Rampasek L, Peacock J, Shih DJH, Luu B et al (2017) Intertumoral heterogeneity within medulloblastoma subgroups. Cancer Cell 12(6):737–754
Louis DN, Ohgaki K, Wiestler OD, Cavenee WK (2016) WHO classification of tumours of the central nervous system. Revised 4th Edition. IARC, Lyon, pp 184–189. ISBN 978-92-832-4492-9
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131:803–820
Cho YJ, Tsherniak A, Tamayo P, Santagata S, Ligon A, GreulichH et al (2011) Integrativegenomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol 29:1424–1430
Huang H, Mahler-Araujo BM, Sankila A, Chimelli L, Yonekawa Y, Kleihues P el al (2000) APC mutations in sporadic medulloblastomas. Am J Pathol 156:433–437
Dahmen RP, Koch A, Denkhaus D, Tonn JC, Sörensen N, Berthold F et a (2001) Deletions of AXIN1, a component of the WNT/wingless pathway, in sporadic medulloblastomas. Cancer Res 61:7039–7043
Koch A, Hrychyk A, Hartmann W, Waha A, Mikeska T, Waha A et al (2007) Mutations of the Wnt antagonist AXIN2 (Conductin) result in TCF-dependent transcription in medulloblastomas. Int J Cancer 121:284–291
Trubicka J, Szperl M, Grajkowska M, Karkucińska-Więckowska A, Tarasińska M, Falana K, Dembowska-Bagińska B, Łastowska M (2016) Identification of a novel inherited ALK variant M1199L in the WNT type of medulloblastoma. Folia Neuropathol 54(1):23–30
Łastowska M, Trubicka J, Niemira M, Paczkowska-Abdulsalam M, Karkucińska-Więckowska A, Kaleta M, Drogosiewicz M, Tarasińska M, Perek-Polnik M, Krętowski A, Dembowska-Bagińska B, Grajkowska W, Pronicki M, Matyja E (2017) ALK expression is a novel marker for the WNT-activated type of pediatric medulloblastoma and an indicator of good prognosis for patients. Am J Surg Pathol 41(6):781–787
Ellison DW, Dalton J, Kocak M, Nicholson SL, Fraga C, Neale G et al (2011) Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol 121:381–396
Łastowska M, Jurkiewicz E, Trubicka J, Daszkiewicz P, Drogosiewicz M, Malczyk K et al (2015) Contrast enhancement pattern predicts poor survival for patients with non-WNT/SHH medulloblastoma tumours. J Neuro Oncol 123(1):65–73
Wang K, Li M, Hakonarson H (2010) ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38(16):e164
Trubicka J, Filipek I, Piotr I, Rydzanicz M, Grajkowska W, Piekutowska-Abramczuk D, Chrzanowska K, Karkucińska-Więckowska A, Iwanicka-Pronicka K, Pronicki M, Łastowska M, Płoski R, Dembowska-Bagińska B (2017) Constitutional mosaicism of a de novo TP53 mutation in a patient with bilateral choroid plexus carcinoma. Cancer Genet 216–217:79–85
Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP (2011) Integrative genomics viewer. Nat Biotechnol 29(1):24–26
Waszak SM, Northcott PA, Buchhalter I, Robinson GW, Sutter C, Groebner S et al (2018) Spectrum and prevalence of genetic predisposition in medulloblastoma: a retrospective genetic study and prospective validation in a clinical trial cohort. Lancet Oncol 19(6):785–798
Acknowledgements
This study was funded by the National Science Centre, Poland, Grant nos. 2016/21/B/NZ2/01785 (M.Ł) and 2016/23/B/NZ2/03064 (J.T).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving participants were in accordance with the ethical standards of the institutional and/or national ethical committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained to use tumor material according to the procedures outlined by the CMHI’s Ethical Committee.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Łastowska, M., Trubicka, J., Karkucińska-Więckowska, A. et al. Immunohistochemical detection of ALK protein identifies APC mutated medulloblastoma and differentiates the WNT-activated medulloblastoma from other types of posterior fossa childhood tumors. Brain Tumor Pathol 36, 1–6 (2019). https://doi.org/10.1007/s10014-018-0331-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10014-018-0331-2