Abstract
There are several strategies for enhancing the sensitivity of electroanalytical methods. Usually, those strategies are based on the selection of the voltammetric technique, the inclusion of an accumulation step, and the eventual addition of a catalytic chemical reaction that regenerates the electroactive species. Square-wave voltammetry (SWV) is one of the most sensitive techniques. In the case of electroanalytical applications, it is typically preceded by an electrochemical or adsorptive pre-concentration step.
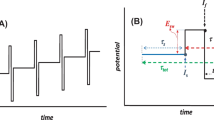
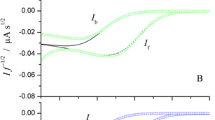
In this manuscript, the theory of SWV for a quasi-reversible electrode process coupled to a catalytic chemical reaction between an adsorbed reagent and a soluble product is presented. The dependences of the dimensionless net peak current and its peak potential on the value of the standard charge transfer rate constant are described. The variation of the SWV parameters such as frequency and potential pulse amplitude are discussed. The effect of the chemical and electrochemical kinetics on the voltammetric profile is analyzed.






Similar content being viewed by others
References
Bobrowski A, Zarebski J (2008) Curr Anal Chem 4:191–201
Bǎnicǎ FG, Ion A (2000) In: Meyers RA (ed) Encyclopaedia of analytical chemistry: instrumentation and applications, vol 12. Wiley, New York, pp. 11115–11143
Czae M, Wang J (1999) Talanta 50:921–928
Mirčeski V, Bobrowski A, Zarebski J, Spasovski F (2010) Electrochim Acta 55:8696–8703
Obata H, van den Berg CMG (2001) Anal Chem 73:2522–2528
Caprara S, Laglera LM, Monticelli D (2015) Anal Chem 87:6357–6363
Espada-Bellido E, Bi Z, van den Berg CMG (2013) Talanta 105:287–291
Vega M, van den Berg CMG (1997) Anal Chem 69:874–881
Abualhaija MM, van den Berg CMG (2014) Mar Chem 164:60–74
Mirčeski V, Quentel F (2005) J Electroanal Chem 578:25–35
O’Dea JJ, Osteryoung J (1986) In: Bard AJ (ed) Square-wave voltammmetry, electroanal chem, Vol 14, Marcel Dekker, New York, pp 209–308
Mirčeski V, Komorsky-Lovrić S, Lovrić M (2007) In: Scholz F (ed) Square-wave voltammertry: theory and application. Heidelberg, Springer Verlag
Mirčeski V, Gulaboski R (2014) Maced J Chem Chem Eng 33:1–12
Garay F (2001) J Electroanal Chem 505:100–108
Garay F (2003) J Electroanal Chem 548:1–9
Lovrić M, Komorsky-Lovrić Š (1988) J Electroanal Chem 248:239–253
Komorsky-Lovrić Š, Lovrić M (1995) J Electroanal Chem 384:115–122
Lovrić M, Komorsky-Lovrić Š, Bond A (1991) J Electroanal Chem 319:1–18
Mirčeski V, Lovrić M (2004) J Electroanal Chem 565:191–202
Laborda E, Suwatchara D, Rees NV, Henstridge MC, Molina A, Compton RG (2013) Electrochim Acta 110:772–779
Garay F, Lovrić M (2002) J Electroanal Chem 518:91–102
Garay F, Lovrić M (2002) Electroanalysis 14:1635–1643
Garay F, Lovrić M (2002) J Electroanal Chem 527:85–92
Mirčeski V, Laborda E, Guziejewski D, Compton RG (2013) Anal Chem 85:5586–5594
Gonzalez J, Molina A, Martinez Ortiz F, Laborda E (2012) J Phys Chem C 116:11206–11215
Chevallier FG, Klymenko OV, Jiang L, Jones TGJ, Compton RG (2005) J Electroanal Chem 574:217–237
Garay F, Solis VM (2001) J Electroanal Chem 505:109–117
Garay F, Solis VM, Lovrić M (1999) J Electroanal Chem 478:17–24
Smith DE (1963) Anal Chem 35:602–609
O’Dea JJ, Osteryoung J, Osteryoung RA (1981) Anal Chem 53:695–701
Zeng J, Osteryoung RA (1986) Anal Chem 58:2766–2771
Mirčeski V, Gulaboski R (2003) J Solid State Electrochem 7:157–165
Nicholson RS, Olmstead M (1972) In: Matson J, Mark H, Macdonald H (eds) Electrochemistry: calculations, simulations and instrumentation, Vol 2 Marcel Dekker, New York, pp 120–137
Bard AJ, Faulkner LR (2001) In: Electrochemical methods, Wiley, New York, pp 813
Garay F, Solis VM (2003) J Electroanal Chem 544:1–11
Mirčeski V, Gulaboski R (2015) Electrochim Acta 167:219–225
Mirčeski V, Skrzypek S, Ciesielski W, Sokołowski A (2005) J Electroanal Chem 585:97–104
Acknowledgments
Financial support from the Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET), Fondo para la Investigación Científica y Tecnológica (FONCYT) and Secretaría de Ciencia y Tecnología de la Universidad Nacional de Córdoba (SECyT-UNC) is gratefully acknowledged. S. V. acknowledges CONICET for the fellowship granted.
Author information
Authors and Affiliations
Corresponding author
Additional information
F. Garay wants to thank to his mentor Prof. Milivoj Lovrić and to Prof. Šebojka Komorsky-Lovrić for all the help and friendship that they gave him since they met. This manuscript is dedicated to them on the occasion of their 65th birthday.
Appendix
Appendix
List of symbols and abbreviations | |
A | Electrode surface |
a | Auxiliary variable of adsorption |
c o | Concentration of oxidized electroactive species |
\( {c}_o^{*} \) | Bulk concentration of oxidized electroactive species |
c r | Concentration of reduced electroactive species |
\( {c}_r^{*} \) | Bulk concentration of reduced electroactive species |
D | Diffusion coefficient |
δ | Time of a numerical integration step |
dE | Potential increment |
E sw | Square-wave amplitude |
E(t) | Dimensioned square-wave potential function |
E°′ | Formal potential of the redox reaction |
E p | Peak potential |
F | Faraday constant |
f | Square-wave frequency |
Γ o | Surface concentration of oxidized species |
\( {\varGamma}_o^{ini} \) | Initial surface concentration of oxidized species |
I(t) | Dimensioned current |
ΔI p | Net peak current |
K ad | Adsorption constant |
k s | Standard charge transfer rate constant |
k cat | Pseudo-first order catalytic rate constant |
\( {k}_{\mathrm{cat}}^{'} \) | Second order catalytic rate constant |
n | Number of exchanged electrons |
φ (t) | Dimensionless potential function |
ϕ | Auxiliary concentration function |
Ψ(t) | Dimensionless current |
ΔΨp | Dimensionless net peak current |
ΔΨ | Dimensionless net current |
Ψ b | Dimensionless backward current |
Ψ f | Dimensionless forward current |
q | Number of subintervals in each wave |
R | Gas constant |
T | Temperature in Kelvin degrees |
t | Time |
θ | Auxiliary concentration function |
x | Distance from the electrode surface |
Rights and permissions
About this article
Cite this article
Vettorelo, S.N., Garay, F. Adsorptive square-wave voltammetry of quasi-reversible electrode processes with a coupled catalytic chemical reaction. J Solid State Electrochem 20, 3271–3278 (2016). https://doi.org/10.1007/s10008-016-3273-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-016-3273-9