Abstract
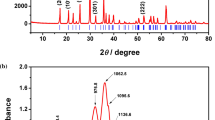
LiFePO4 samples have been synthesized by mixing stoichiometric amounts of (NH4)2HPO4, FeC2O4·2H2O, and LiF. During synthesis, carbon gel was used as the carbon source. Single-phase LiFePO4 can be formed when the heating temperature ranges from 650 to 800 °C and it is decomposed into Li4P2O7, Li3PO4, Fe2P, and Li3P7 when the temperature comes to 850 °C. We find that the ratio of the lattice parameter (a/c) decreases with the increasing temperature, thereby increasing the Li+ diffusion channel length. Both the decrease of a/c and the abrupt crystal growth are expected to contribute to the monotonic decrease of the initial capacity of the samples. The sample heated at 650 °C with a smaller uniform particle size and relative higher specific surface area (8.2 m2/g) shows an excellent electrochemical performance. The initial specific capacity of 156.7(3) mAh/g is obtained at the rate of C/10.









Similar content being viewed by others
References
Thackeray MM, Thomas JO, Whittingham MS (2000) MRS Bull 25(3):39
Kerr TA, Gaubicher J, Nazar LF (2000) Electrochem Solid-State Lett 3:460. doi:10.1149/1.1391179
Chung SY, Bloking JT, Chiang YM (2002) Nat Mater 1:123. doi:10.1038/nmat732
Franger S, Cras FL, Bourbon C, Rouault H (2002) Electrochem Solid-State Lett 5:231. doi:10.1149/1.1506962
Takahashi M, Tobishima S, Takei K, Sakurai Y (2001) J Power Sources 97–98:508. doi:10.1016/S0378-7753(01) 00728-5
Deoff MM, Hu YQ, McLarnon F, Kostecki R (2003) Electrochem Solid-State Lett 6:207. doi:10.1149/1.1601372
Huang H, Yin SC, Nazar LF (2001) Electrochem Solid-State Lett 4:170. doi:10.1149/1.1396695
Franger S, Bourbon C, Cras FL (2004) J Electrochem Soc 151:1024. doi:10.1149/1.1758721
Prosini PP, Zane D, Pasquali M (2001) Electrochim Acta 46:3517. doi:10.1016/S0013-4686(01) 00631-4
Park KS, Son JT, Chung HT, Kim SJ, Lee CH, Kang KT, Kim HG (2004) Solid State Commun 129:311. doi:10.1016/j.ssc.2003.10.015
Wang GX, Bewlay S, Yao J, Ahn JH, Liu HK, Dou SX (2004) Electrochem Solid-State Lett 7:A503. doi:10.1149/1.1819867
Yamada A, Chung SC, Hinokuma K (2001) J Electrochem Soc 148:A224. doi:10.1149/1.1348257
Li G, Azuma H, Tohda M (2002) J Electrochem Soc 149:A743. doi:10.1149/1.1473776
Spong AD, Vitins G, Owen JR (2005) J Electrochem Soc 152:A2376. doi:10.1149/1.2120427
Wang D, Li H, Wang Z, Wu X, Sun Y, Huang X, Chen L (2004) J Solid State Chem 177:4582. doi:10.1016/j.jssc.2004.09.013
Lin C, Ritter JA (1997) Carbon 35(9):1271. doi:10.1016/S0008-6223(97)00069-9
Franger S, Cras FL, Bourbon C, Rouault H (2003) J Power Sources 119–121:252. doi:10.1016/S0378-7753(03) 00242-8
Cho TH, Chung HT (2004) J Power Sources 133:272. doi:10.1016/j.jpowsour.2004.02.015
Ouyang CY, Shi SQ, Wang ZX, Huang XJ, Chen LQ (2004) Phys Rev B 69(10):104303
Andersson AS, Thomas JO (2001) J Power Sources 97–98:498. doi:10.1016/S0378-7753(01) 00633-4
Barker J, Saidi MY, Swoyer JL (2004) J Electrochem Soc 151:A1670. doi:10.1149/1.1785796
Barker J, Saidi MY, Swoyer JL (2003) Electrochem Solid-State Lett 6:A53. doi:10.1149/1.1544211
Barker J, Saidi MY, Swoyer JL (2003) J Electrochem Soc 150:A684. doi:10.1149/1.1568936
Acknowledgements
This work was supported by NSFC Grant (No. 50772039) and by the Science and Technology Bureau of Guangdong government (No. 07118058). The authors are indebted to Prof. G. L. Lv of Zhejiang University of China and Prof. C. Dong of Institute of Physics, Chinese Academy of Science for their assistance with the XRD experiments. We also thank Prof. Y. J. Zhao and Prof. X. Chen of South China University of Technology for the correction on the language.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, Y.Z., Zhao, Y.M., Duan, H. et al. Electrochemical properties of single-phase LiFePO4 synthesized using LiF as Li precursor and hydrogen and carbon gel as reducing agents. J Solid State Electrochem 14, 131–137 (2010). https://doi.org/10.1007/s10008-009-0798-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-009-0798-1