Abstract
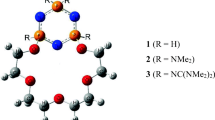
The interactions between crown ether ligands (14-crown-4, 14C4; 4,4,5,5-tetramethylbenzo-14-crown-4, BC4H12-14C4; 4,4,5,5,9,9,10,10-octamethyl-14-crown-4, C8H24-14C4; dibenzo-14-crown ether-4, DB14C4) and alkaline and alkaline earth metal ions (Li+, Na+, Mg2+) were investigated using density functional theory modeling at the M062X/def2SVP and def2TZVP level. The condensed softness analysis of crown ethers, a condensed Fukui function, a condensed dual descriptor, and frontier molecular orbital theory were used to analyze the reactivities of the complexes. The complex stability was analyzed in terms of the binding energies, standard Gibbs free energy of formation, and energy decomposition of the interaction in aqueous solution. The results show that the active sites were mainly located at the carbon atoms of the benzene ring and oxygen atoms. The reactivities of DB14C4 and BC4H12-14C4 are higher than those of 14C4 and C8H24-14C4. The electrostatic interaction is the principal factor determining the stability of the complexes. The complexes containing Li+ has the greatest stability in aqueous solution among the complexes containing Li+, Na+, and Mg2+. BC4H12-14C4 shows selective adsorption toward Li+ in a mixed solution of Li+, Na+, and Mg2+. To evaluate the stability of complexes containing Mg2+, the solvent effect must be accurately described. An energy decomposition analysis was used to evaluate the stability of complexes containing Li+, Na+, and Mg2+, and the solvent effects were considered.





Similar content being viewed by others
References
Faulkner S, Kataky R, Parker D, Teasdale A (1995) Lithium selective ionophores based on pendant arm substituted crown ethers. J Chem Soc Perkin Trans 2:1761–1769
Kamenica M, Kothur RR, Willows A, Patel BA, Cragg PJ (2017) Lithium ion sensors. Sensors 17:2430–2447
Torrejos REC, Nisola GM, Park MJ, Shon HK, Seo JG, Koo S (2015) Synthesis and characterization of multi-walled carbon nanotubes-supported dibenzo-14-crown-4 ether with proton ionizable carboxyl sidearm as Li+ adsorbents. Chem Eng J 264:89–98
Alexandratos SD, Stine CL, Sachleben RA, Moyer BA (2005) Immobilization of lithium-selective 14-crown-4 on crosslinked polymer supports. Polymer 46:6347–6352
An JW, Kang DJ, Tran KT, Kim MJ, Lim T, Tran T (2012) Recovery of lithium from Uyuni salar brine. Hydrometallurgy 117:64–70
Chung K, Lee J, Kim W, Kim S, Cho K (2008) Inorganic adsorbent containing polymeric membrane reservoir for the recovery of lithium from seawater. J Membr Sci 325:503–508
Hu S, Sun Y, Pu M, Yun R, Xiang X (2019) Determination of boundary conditions for highly efficient separation of magnesium and lithium from salt lake brine by reaction-coupled separation technology. Sep Purif Technol 229:115813
Li X, Mo Y, Qing W, Shao S, Tang CY, Li J (2019) Membrane-based technologies for lithium recovery from water lithium resources: a review. J Membr Sci 591:117317
Sun YZ, Zhao C, Zhang J (2013) Concentrations of valuable elements of the coals from the Pingshuo Mining District, Ningwu Coalfield, northern China. Energy Explor Exploit 31:727–744
Swain B (2016) Recovery and recycling of lithium: a review. Sep Purif Technol 172:388–403
Hua Z, Geng A, Tang Z, Zhao Z, Liu H, Yao Y, Yang Y (2019) Decomposition behavior and reaction mechanism of Ce0.67Tb0.33MgAl11O19 during Na2CO3 assisted roasting: toward efficient recycling of Ce and Tb from waste phosphor. J Environ Manag 249:109383
Zhao Z, Yang Y, Xiao Y, Fan Y (2012) Recovery of gallium from Bayer liquor: a review. Hydrometallurgy 125:115–124
Mei Q, Tian R, Shi Y, Hua Q, Chen C, Tong B (2016) A series of selective and sensitive fluorescent sensors based on a thiophen-2-yl-benzothiazole unit for hg 2+. New J Chem 40:2333–2342
Yu HL, Wang WY, Hong B, Zong Y, Si YL, Hu ZQ (2016) Variational first hyperpolarizabilities of 2, 3-naphtho-15-crown-5 ether derivatives with cation-complexing: a potential and selective cation detector. Phys Chem Chem Phys 18:26487–26494
López JC, Pérez C, Blanco S, Shubert VA, Temelso B, Shields GC, Schnell M (2019) Water induces the same crown shapes as Li+ or Na+ in 15-crown-5 ether: a broadband rotational study. Phys Chem Chem Phys 21:2875–2881
Ueno K, Tatara R, Tsuzuki S, Saito S, Doi H, Yoshida K, Watanabe M (2015) Li+ solvation in glyme-Li salt solvate ionic liquids. Phys Chem Chem Phys 17:8248–8257
Hurtado P, Hortal AR, Gámez F, Hamad S, Martínez-Haya B (2010) Gas–phase complexes of cyclic and linear polyethers with alkali cations. Phys Chem Chem Phys 12:13752–13758
Torrejos REC, Nisola GM, Song HS, Han JW, Lawagon CP, Seo JG, Koo S, Kim H, Chung WJ (2016) Liquid-liquid extraction of lithium using lipophilic dibenzo-14-crown-4 ether carboxylic acid in hydrophobic room temperature ionic liquid. Hydrometallurgy 164:362–371
Hou H, Zeng X, Liu X (2009) DFT study of a series of crown-4 ethers and their selectivity trend for alkali metal cations: Li+ and Na+. J Mol Model 15:105–111
Bartsch RA, Czech BP, Kang S, Stewart LE, Walkowiak W, Charewicz WA, Heo GS, Son B (1985) High lithium selectivity in competitive alkali metal solvent extraction by lipophilic crown carboxylic acids. J Am Chem Soc 107:4997–4998
Inoue Y, Hakushi T, Yu L, Tong LH (1993) Molecular design of crown ethers. 12. Complexation thermodynamics of 12-to 16-crown-4: thermodynamic origin of high lithium selectivity of 14-crown-4. J Organomet Chem 58:5411–5413
Torrejos REC, Nisola GM, Song HS, Limjuco LA, Lawagon CP, Parohinog KJ, Chung WJ (2017) Design of lithium selective crown ethers: synthesis, extraction and theoretical binding studies. Chem Eng J 326:921–933
Walkowiak W, Brown PR, Shukla JP, Bartsch RA (1987) Selective separation of alkali metal cations by bulk chloroform membranes containing lipophilic crown ether phosphonic acid monoethyl esters. J Membr Sci 32:59–68
Liu Y, Inoue Y, Hakushi (1990) T. molecular design of crown ethers. VII. Syntheses and cation selectivities of unsubstituted 12-to 16-Crown-4. Bull Chem Soc Jpn 63: 3044–3046
Czech BP, Babb DA, Son B, Bartsch RA (1984) Functionalized 13-crown-4, 14-crown-4, 15-crown-4, and 16-crown-4 compounds: synthesis and lithium ion complexation. J Organomet Chem 49:4805–4810
Sachleben RA, Davis MC, Bruce JJ, Ripple ES, Driver JL, Moyer BA (1993) An efficient synthesis of lithium-selective extractants: tertiary-alkyl-14-crown-4 ethers. Tetrahedron Lett 34:5373–5376
Lu T, Chen F (2012) Multiwfn: a multifunctional wave function analyzer. J Comput Chem 33:580–592
Cao J, Ren Q, Chen F, Lu T (2015) Comparative study on the methods for predicting the reactive site of nucleophilic reaction. Sci China Chem 58:1845–1852
Ho J, Klamt A, Coote ML (2010) Comment on the correct use of continuum solvent models. J Phys Chem A 114:13442–13444
Achazi AJ, von Krbek LKS, Schalley CA, Paulus B (2016) Theoretical and experimental investigation of crown/ammonium complexes in solution. J Comput Chem 37:18–24
Golebiowski J, Lamare V, Ruiz López MF (2002) Rb+/Cs+ selectivity of benzo and tribenzo derivatives of the 21C7 crown ether. A density functional study. J Comput Chem 23:724–731
Parr RG, Yang W (1984) Density functional approach to the frontier-electron theory of chemical reactivity. J Am Chem Soc 106:4049–4050
Morell C, Grand A, ToroLabbé A (2005) New dual descriptor for chemical reactivity. J Phys Chem A 109(1):205–212
Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz KM, Ferguson DM (1995) A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. J Am Chem Soc 117:5179–5197
Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA (2004) Development and testing of a general amber force field. J Comput Chem 25:1157–1174
Lu T, Multiwfn Manual, version 3.6(dev), Section 4.100.8, available at http://sobereva.com/multiwfn (accessed Aug 30, 2018).
Fu R, Lu T, Chen FW (2014) Comparing methods for predicting the reactive site of electrophilic substitution. Acta Phys -Chim Sin 30:628–639
Fleming I (1977) Frontier orbital and organic chemical reactions. Wiley, New Jersey
Azizoglu A (2003) Quantum chemical investigation of monostanna[n]cyclacenes. Struct Chem 14:575–580
Kruse H, Grimme S (2012) A geometrical correction for the inter- and intra-molecular basis set superposition error in Hartree-Fock and density functional theory calculations for large systems. J Chem Phys 136:154101–154117
Cramer CJ (2013) Essentials of computational chemistry: theories and models. John Wiley & Sons, New Jersey
Kim KS, Tarakeshwar P, Lee JY (2000) Molecular clusters of π-systems: theoretical studies of structures, spectra, and origin of interaction energies. Chem Rev 100:4145–4186
Hintze KJ, Lützen A, Bredow T (2015) Structure and stability of supramolecular crown ether complexes. J Comput Chem 36:1467–1472
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566
Kavimani M, Balachandran V, Narayana B, Vanasundari K, Revathi B (2018) Topological analysis (BCP) of vibrational spectroscopic studies, docking, RDG, DSSC, Fukui functions and chemical reactivity of 2-methylphenylacetic acid. Spectrochim Acta A 190:47–60
Shainyan BA, Chipanina NN, Aksamentova TN, Oznobikhina LP, Rosentsveig GN, Rosentsveig IB (2010) Intramolecular hydrogen bonds in the sulfonamide derivatives of oxamide, dithiooxamide, and biuret. FT-IR and DFT study, AIM and NBO analysis. Tetrahedron 66:8551–8556
Lu T, Chen FW (2011) Calculation of molecular orbital composition. Acta Chim Sin 69:2393–2406
Johnson ER, Keinan S, Mori-Sánchez P, Contreras-García J, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc 132:6498–6506
Lefebvre C, Rubez G, Khartabil H, Boisson JC, ContrerasGarcía J, Hénon E (2017) Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. Phys Chem Chem Phys 19:17928–17936
Zhou F, Liu Y, Wang Z, Lu T, Yang Q, Liu Y, Zheng B (2019) A new type of halogen bond involving multivalent astatine: an ab initio study. Phys Chem Chem Phys 21:15310–15318
Liu JR (2007) Theoretical and experimental study of DB14C4 and its complexes, Doctoral dissertation. Cheng Du University of Technology, China
Kimura K, Yano H, Kitazawa S, Shono T (1986) Synthesis and selectivity for lithium of lipophilic 14-crown-4 derivatives bearing bulky substituents or an additional binding site in the side arm. J Chem Soc Perkin Trans 2:1945–1951
Funding
Contract/grant sponsor: National Natural Science Foundation of China; contract/grant number: 51704011, 21572001, 51904003.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• M062X/def2TZVP is the proper level to calculate the Gibbs free energy of formation of these complexes composed of crown ethers and Li+, Na+, and Mg2+ metal ions with a small BSSE component.
• The interactions between crown ethers and Li+, Na+, and Mg2+ ions mainly include electrostatic, dispersion, and repulsion interactions. Electrostatic interactions are principal factors in stabilizing the complexes. The interactions are also visualized using RDG and IGM functions.
• The electrophilic reactivities of BC4H12-14C4 and DB14C4 are higher than those of C8H24-14C4 and 14C4 because of the presence of a benzene ring. The stability in aqueous solutions of complexes containing Li+ is the greatest among the complexes containing Li+, Na+, and Mg2+. BC4H12-14C4 shows selective adsorption toward Li+ in the mixed solution containing Li+, Na+, and Mg2+, compared with C8H24-14C4, 14C4, and DB14C4.
• Energy decomposition analysis can qualitatively evaluate the stability of complexes containing Li+, Na+, and Mg2+ in aqueous solution when the solvent effect is considered. The key factor to evaluate the stability of complexes containing Mg2+ is whether the solvent effect can be accurately described.
Electronic supplementary material
ESM 1
(DOCX 5069 kb)
Rights and permissions
About this article
Cite this article
Tian, Y., Chen, W., Zhao, Z. et al. Interaction and selectivity of 14-crown-4 derivatives with Li+, Na+, and Mg2+ metal ions. J Mol Model 26, 67 (2020). https://doi.org/10.1007/s00894-020-4325-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-4325-8