Abstract
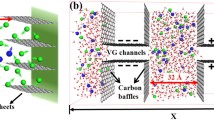
In this study, molecular dynamics (MD) simulations have been performed to explore the variation of ion density and electric potential due to electrode surface modification. Two different surface morphologies, having planer and slit pore with different conditions of surface charge, have been studied for graphene-MnO2 surface using LAMMPS. For different pore widths, the concentration of ions in the double layer is observed to be very low when the surface of the graphene-MnO2 electrode is charged. With a view to identify the optimal pore size for the simulation domain considered, three different widths for the nano-slit type pores and the corresponding ion-ion interactions are examined. Though this effect is negligible for pores with 9.23 and 3.55 Å widths, a considerable increase in the ionic concentration within the 7.10 Å pores is observed when the electrode is kept neutral. The edge region of these nano-slit pores leads to effective energy storage by promoting ion separation and a significantly higher charge accumulation is found to occur on the edges compared to the basal planes. For the simulation domain of the present study, partition coefficient is maximum for a pore size of 7.10 Å, indicating that the ions’ penetration and movement into nano-slit pores are most favorable for this optimum pore size for MnO2-graphene electrodes with aqueous NaCl electrolyte.

The importance of understanding the commercial feasibility of supercapacitor material has made qualitatively predicting the optimized electrode structure one of the main targets of energy related researches. While great progress has been made in recent years, a coherent theoretical picture of the optimized electrode structure remains elusive. This article discusses the most favorable design of supercapacitor electrode for ion-electrode interaction.








Similar content being viewed by others
References
Simon P, Gogotsi Y (2008) Materials for electrochemical capacitors. Nat Mater 7(11):845–854
Miller JR, Simon P (2008) Electrochemical capacitors for energy management. Science. 321(5889):651–652
Zheng JP (2005) Theoretical energy density for electrochemical capacitors with intercalation electrodes. J Electrochem Soc 152(9):A1864–A1869
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric Field effect in atomically thin carbon films. Science. 306(5696):666–669
Stoller MD, Park S, Zhu Y, An J, Ruoff RS (2008) Graphene-based ultracapacitors. Nano Lett 8(10):3498–3502
Xia J, Chen F, Li J, Tao N (2009) Measurement of the quantum capacitance of graphene. Nat Nanotechnol 4(8):505–509
Kuamit T, Ratanasak M, Rungnim C, Parasuk V (2017) Effects of shape, size, and pyrene doping on electronic properties of graphene nanoflakes. J Mol Model 23(12):355
Herath D, Dinadayalane T (2017) Computational investigation of double nitrogen doping on graphene. J Mol Model 24(1):26
Zhai Y, Dou Y, Zhao D, Fulvio PF, Mayes RT, Dai S (2011) Carbon materials for chemical capacitive energy storage. Adv Mater 23(42):4828–4850
Liu R, Wen D, Zhang X, Wang D, Yang Q, Yuan B, Lü W (2018) Three-dimensional reduced-graphene/MnO2 prepared by plasma treatment as high-performance supercapacitor electrodes. Mater Res Express 5(6):065504
Jaggi N, Sharma D, Sharma P (2016) MnO2/PVP/MWCNT hybrid nano composites as electrode materials for high performance supercapacitor. Mater Res Express 3(10):105503
Dong X, Shen W, Gu J, Xiong L, Zhu Y, Li H, Shi J (2006) MnO2-embedded-in-mesoporous-carbon-wall structure for use as electrochemical capacitors. J Phys Chem B 110(12):6015–6019
Liang M, Luo B, Zhi L (2009) Application of graphene and graphene-based materials in clean energy-related devices. Int J Energy Res 33(13):1161–1170
Chmiola J, Largeot C, Taberna P-L, Simon P, Gogotsi Y (2008) Desolvation of ions in subnanometer pores and its effect on capacitance and double-layer theory. Angew Chem Int Ed 47(18):3392–3395
Alibalazadeh M, Foroutan M (2015) Specific distributions of anions and cations of an ionic liquid through confinement between graphene sheets. J Mol Model 21(7):168
Feng G, Qiao R, Huang J, Sumpter BG, Meunier V (2010) Atomistic insight on the charging energetics in subnanometer pore supercapacitors. J Phys Chem C 114(41):18012–18016
Yang H, Zhang X, Yang J, Bo Z, Hu M, Yan J, Cen K (2017) Molecular origin of electric double-layer capacitance at multilayer graphene edges. J Phys Chem Lett 8(1):153–160
Yang H, Yang J, Bo Z, Zhang S, Yan J, Cen K (2016) Edge effects in vertically-oriented graphene based electric double-layer capacitors. J Power Sources 324:309–316
Yike H, Xiaohong L, Shu L, Tianying Y (2016) Development of mean-field electrical double layer theory. Chinese Physics B 25(1):016801
Wang Z, Yang Y, Olmsted DL, Asta M, Laird BB (2014) Evaluation of constant potential method in simulating electric double-layer capacitors. J Chem Phys 141(18):184102
Noh C, Jung Y (2019) Understanding the charging dynamics of an ionic liquid electric double layer capacitor via molecular dynamics simulations. Phys Chem Chem Phys 21(13):6790–6800
Jiang G, Cheng C, Li D, Liu JZ (2016) Molecular dynamics simulations of the electric double layer capacitance of graphene electrodes in mono-valent aqueous electrolytes. Nano Res 9(1):174–186
Rajput NN, Monk J, Singh R, Hung FR (2012) On the influence of pore size and pore loading on structural and dynamical heterogeneities of an ionic liquid confined in a slit nanopore. J Phys Chem C 116(8):5169–5181
Salemi S, Akbarzadeh H, Abdollahzadeh S (2016) Nano-confined ionic liquid [emim][PF6] between graphite sheets: a molecular dynamics study. J Mol Liq 215:512–519
Chai Y, Li Z, Wang J, Mo Z, Yang S (2019) Construction of hierarchical holey graphene/MnO2 composites as potential electrode materials for supercapacitors. J Alloys Compd 775:1206–1212
Striolo A (2011) From interfacial water to macroscopic observables: a review. Adsorpt Sci Technol 29(3)
Kalluri RK, Konatham D, Striolo A (2011) Aqueous NaCl solutions within charged carbon-slit pores: partition coefficients and density distributions from molecular dynamics simulations. J Phys Chem C 115(28):13786–13795
Jorgensen WL (1981) Quantum and statistical mechanical studies of liquids. 10. Transferable intermolecular potential functions for water, alcohols, and ethers. Application to liquid water. J Am Chem Soc 103(2):335–340
Alexiadis A, Kassinos S (2008) Molecular simulation of water in carbon nanotubes. Chem Rev 108(12):5014–5034
MacKerell AD, Bashford D, Bellott M, Dunbrack RL, Evanseck JD, Field MJ, Fischer S, Gao J, Guo H, Ha S, Joseph-McCarthy D, Kuchnir L, Kuczera K, Lau FTK, Mattos C, Michnick S, Ngo T, Nguyen DT, Prodhom B, Reiher WE, Roux B, Schlenkrich M, Smith JC, Stote R, Straub J, Watanabe M, Wiórkiewicz-Kuczera J, Yin§M. Karplus D. (1998) All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B 102(18):3586–3616
Miyamoto S, Kollman PA (1992) Settle: An analytical version of the SHAKE and RATTLE algorithm for rigid water models. J Comput Chem 13(8):952–962
Plimpton S (1995) Fast parallel algorithms for short-range molecular dynamics. J Comput Phys 117:1–19
Allen MP, Allen MP, Tildesley DJ, Tildesley DJ (1989) Computer simulation of liquids. Clarendon press 412 p
Nosé S (1984) A unified formulation of the constant temperature molecular dynamics methods. J Chem Phys 81(1):511–519
Hoover WG (1985) Canonical dynamics: equilibrium phase-space distributions. Phys Rev A 31(3):1695–1697
Wander MCF, Shuford KL (2010) Molecular dynamics study of interfacial confinement effects of aqueous NaCl brines in nanoporous carbon. J Phys Chem C 114(48):20539–20546
Panteva MT, Giambaşu GM, York DM (2015) Force field for Mg2+, Mn2+, Zn2+ and Cd2+ ions that have balanced interactions with nucleic acids. J Phys Chem B 119(50):15460–15470
Mark P, Nilsson L (2001) Structure and dynamics of the TIP3P, SPC, and SPC/E water models at 298 K. J Phys Chem A 105(43):9954–9960
Chen M, Li S, Feng G (2017) The influence of anion shape on the electrical double layer microstructure and capacitance of ionic liquids-based supercapacitors by molecular simulations. Molecules. 22(2):241
Li S, Feng G, Cummings PT (2014) Interfaces of dicationic ionic liquids and graphene: a molecular dynamics simulation study. J Phys Condens Matter 26(28):284106
Yan J, Fan Z, Wei T, Qian W, Zhang M, Wei F (2010) Fast and reversible surface redox reaction of graphene–MnO2 composites as supercapacitor electrodes. Carbon. 48(13):3825–3833
Lian C, Liu K, Van Aken KL, Gogotsi Y, Wesolowski DJ, Liu HL, Jiang DE, Wu JZ (2016) Enhancing the capacitive performance of electric double-layer capacitors with ionic liquid mixtures. ACS Energy Lett 1(1):21–26
Szewczyk A, Sikula J, Sedlakova V, Majzner J, Sedlak P, Kuparowitz T (2016) Voltage dependence of supercapacitor capacitance. Metrol Meas Syst 23(3):403–411
Vatamanu J, Cao L, Borodin O, Bedrov D, Smith GD (2011) On the influence of surface topography on the electric double layer structure and differential capacitance of graphite/ionic liquid interfaces. J Phys Chem Lett 2(17):2267–2272
Kalluri RK, Biener MM, Suss ME, Merrill MD, Stadermann M, Santiago JG, Baumann TF, Biener J, Striolo A (2013) Unraveling the potential and pore-size dependent capacitance of slit-shaped graphitic carbon pores in aqueous electrolytes. Phys Chem Chem Phys 15(7):2309–2320
Xi YH, Liu Z, Ji J, Wang Y, Faraj Y, Zhu Y, Xie R, Ju XJ, Wang W, Lu X, Chu LY (2018) Graphene-based membranes with uniform 2D nanochannels for precise sieving of mono-/multi-valent metal ions. J Membr Sci 550:208–218
Yuan W, Zhou Y, Li Y, Li C, Peng H, Zhang J, Liu Z, Dai L, Shi G (2013) The edge- and basal-plane-specific electrochemistry of a single-layer graphene sheet. Sci Rep 3:2248
Acknowledgments
This work was supported by providing allocations of computing by the Institute of Information and Communication Technology (IICT), BUET and by the Post Graduate Lab (Department of Mechanical Engineering, BUET). The authors declare no competing financial interest.
Funding
The project is partially funded by Committee for Advanced Studies and Research (CASR), BUET.
Author information
Authors and Affiliations
Contributions
This publication is a part of M.Sc. thesis of Musanna Galib under supervision of Md. Ashiqur Rahman and Shakhawat H. Firoz with suggestions and guidelines from Mohammad Mozammal Hosen, Joyanta K. Saha, Md. Mominul Islam. All authors have given approval to the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Code availability
Code is not available due to privacy restrictions.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Galib, M., Hosen, M.M., Saha, J.K. et al. Electrode surface modification of graphene-MnO2 supercapacitors using molecular dynamics simulations. J Mol Model 26, 251 (2020). https://doi.org/10.1007/s00894-020-04483-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04483-5