Abstract
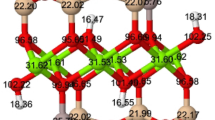
Muscovite (Ms) and phlogopite (Phl) belong to the 2:1 dioctahedral and trioctahedral layer silicates, respectively, and are the end members of Ms-Phl series minerals. This series was studied in the 2M1 polytype and modeled by the substitution of three Mg2+ cations in the Phl octahedral sites by two Al3+ and one vacancy, increasing the substitution up to reach the Ms. The series was computationally examined at DFT level as a function of pressure to 9 GPa. Cell parameters as a function of pressure and composition, and bulk moduli as a function of the composition agrees with the existing experimental results. The mixing Gibbs free energy was calculated as a function of composition. From these data, approximated solvi were calculated at increasing pressure. A gap of solubility is found, decreasing the gap of solubility at high pressure.









Similar content being viewed by others
References
Huang WL, Wyllie PJ (1973) Muscovite dehydratation and melting in deep crust and subducted oceanic sediments. Earth Planet Sci Lett 18:133–136
Hwang H, Seoung D, Lee Y, Liu Z, Liermann HL, Cynn H, Vogt T, Kao C-C, Mao H-K (2017) A role for subducted super-hydrated kaolinite in Earth’s deep water cycle. Nat Geosci 10:947–953
Schmidt M, Poli S (1998) Experimentally based water budgets for dehydrating slabs and consequences for arc magma generation. Earth Planet Sci Lett 163:361–379
Schmidt M, Wielzeuf D, Auzanneau E (2004) Melting and dissolution of subducting crust at high pressures: the key role of white mica. Earth Planet Sci Lett 228:65–84
Robert J-L (1976) Phlogopite solid solutions in the system K2O-MgO-Al2O3-SiO2-H2O. Chem Geol 17:195–212
Monier G, Robert J-L (1986) Muscovite solid solutions in the system K2O-MgO-FeO-Al2O3-SiO2-H2O: an experimental study at 2 kbar PH2O and comparison with natural li-free white micas. Mineral Mag 50:257–266
Rutherford MJ (1973) The phase relations of aluminous iron biotites in the system KAlSi3O8-KAlSiO4-Al2O3-Fe-O-H. J Petrol 14:159–180
Foster MD (1960) Interpretation of the composition of trioctahedral micas. US Geol Surv Prof Pap 354:8, 48 pp
Crowley MS, Roy R (1964) Crystalline solubility in muscovite and phlogopite groups. Am Mineral 49:348
Faust J, Knittle E (1994) The equation of state, amorphization and high pressure phase diagram of muscovite. J Geophys Res 99:19785–19792
Mookherjee M, Steinle-Neumann G (2009) Deeply subducted crust from the elasticity of hollandite. Earth Planet Sci Lett 288:349–358
Sekine T, Rubin AM, Ahrens TJ (1991) Shock wave equation of state of muscovite. J Geophys Res 96:19675–19680
Domanik KJ, Holloway JR (1996) The stability and composition of phengitic muscovite and associated phases from 5.5 to 11 GPa. Implications for deeply subducted sediments. Geochim Cosmochim Acta 60:4133–4150
Domanik KJ, Holloway JR (2000) Experimental synthesis and phase relations of phengitic muscovite from 6.5 to 11 GPa in a calcareous methapelite from the Dabie mountains, China. Lithos 52:51–77
Yoder HS, Eugster HP (1954) Phlogopite synthesis and stability range. Geochim Cosmochim Acta 6:167–185
Yoder HS, Kushitro I (1969) Melting of a hydrous phase: phlogopite. Am J Sci 267(A):558–583
Kushiro I, Akimoto S, Syono Y (1967) Stability of phlogopite at high pressure and possible presence of phlogopite in the earth’s upper mantle. Earth Planet Sci Lett 3:197–203
Trønnes RG (2002) Stability range and decomposition of potassic richterite and phlogopite end member at 5-15 GPa. Mineral Petrol 74:129–148
Sato K, Katsura T, Ito E (1997) Phase relations of natural phlogopite with and without enstatite up to 8 GPa: implications for the mantle metasomatism. Earth Planet Sci Lett 146:511–526
Wyllie PJ, Sekine T (1982) Formation of mantle phlogopite in subduction zone hybridization. Contrib Mineral Petrol 79:375–380
Gill J (1981) Orogenic andesites and plate tectonics. Springer-Verlag, p 390
Hazen RM, Finger LW (1978) The crystal structures and compressibilities of layer minerals at high pressure. II Phlogopite and Chlorite. Am Mineral 63:293–296
Ortega-Castro J, Hernández-Haro N, Timón V, Sainz-Diaz CI, Hernández-Laguna A (2010) High-pressure behaviour of 2M1 muscovite. Am Mineral 95:249–259. https://doi.org/10.2138/am.2010.3035
Ullan G, Valdrè G (2015) Density functional investigation of the thermos-physical and thermo-chemical properties of 2M 1 muscovite. Am Mineral 100:935–944
Chheda TD, Mookherjee M, Mainprice D, dos Santos AM, Molaison JJ, Chantel J, Manthilake G, Bassett WA (2014) Structure and elasticity of phlogopite under compression: geophysical implications. Phys Earth Planet Inter 233:1–12
Hernández-Haro N, Muñoz-Santiburcio D, Pérez del Valle C, Ortega-Castro J, Sainz-Díaz CI, Garrido CJ, Hernández-Laguna A (2016) Computational study of pressure behaviour to 6 GPa of the 2M1 muscovite-paragonite series. Am Mineral 101:1207–1216
Hernández-Haro N, Ortega-Castro J, Pérez del Valle C, Muñoz-Santiburcio D, Sainz-Díaz CI, Hernández-Laguna A (2013) Computational study of the elastic behaviour of the 2M1 muscovite-paragonite series. Am Mineral 86:651–664
Hernández-Haro N, Ortega-Castro J, Pruneda M, Sainz-Díaz CI, Hernández-Laguna A (2014) Theoretical study on the influence of the Mg2+ and Al3+ octahedral cations on the vibrational spectra of hydroxyl groups of 2:1 dioctahedral phyllosilicate models. J Mol Model 20:2402, 10 pages
Escamilla-Roa E, Hernández-Laguna A, Sainz-Díaz CI (2013) Cation arrengement in the octahedral and tetrahedral sheets of cis vacant polymorph of dioctahedral 2:1 phyllosilicates by quantum mechanical calculations. Am Mineral 98:724–735
Briones-Jurado C, Agacino-Valdés E (2009) BrOnsted sites on acid-treated montmorillonites: a theoretical study with probe molecules. J Phys Chem A 113:8994–9001
Wang Q, Zhu C, Yun J, Yang G (2017) Isomorphic substitutions in clay materials and adsorption of metals onto external surfaces: a DFT investigation. J Phys Chem C 121:26722–26732
Escamilla-Roa E, Huertas FJ, Hernández-Laguna A, Sainz-Díaz CI (2017) A DFT study of the adsorption of glycine in the interlayer space of montmorillonite. Phys Chem Chem Phys 19:14961–14971
Wang Q, Zhu C, Yun J, Hu Q, Yang G (2018) Compositional transformations as well as thermodynamics and mechanism of dissolution for clay minerals. Chem Geol 494:109–116
Sainz-Díaz CI, Escamilla-Roa E, Hernández-Laguna A (2004) Pyrophyllite dehydroxylation process by first principle calculations. Am Mineral 69:1092–1100
Palin EJ, Dove MT, Redfern SAT, Ortega-Castro J, Sainz-Díaz CI, Hernández-Laguna A (2014) Computer simulations of cations order-disorder in 2:1 dioctahedral phyllosilicates using cation-exchange potentials and Monte Carlo methods. Int J Quantum Chem:114:1257–1286. https://doi.org/10.1002/qua.24703
Bailey SW (1984) Crystal chemistry of the true micas. Mineral Soc Am Rev Mineral 13:13–60
Sánchez-Portal D, Ordejón P, Artacho E, Soler JM (1997) Density-functional method for very large systems with LCAO basis sets. Int J Quantum Chem 65:453–461
Artacho E, Sánchez-Portal D, Ordejón P, García A, Soler JM (1999) Linear-scaling ab-initio calculations for large and complex systems. Phys Status Solidi B 215:809–817
Soler JM, Artacho E, Gale JD, García A, Junquera J, Ordejón P, Sánchez-Portal D (2002) The SIESTA method for ab-initio order-N materials simulation. J Phys Condens Matter 14:2745–2779
Giannozzi P, Baroni S, Bonini, N., Calandra, M., Car, R., Cavazzoni, C., Ceresoli, D., Chiarotti GL, Cococcioni M, Dabo I, Dal Corso A, de Gironcoli S, Fabris S, Fratesi G, Gebauer R, Gerstmann U, Gougoussis C, Kokalj A, Lazzeri M, Martin-Samos L, Marzari N, Mauri F, Mazzarello R, Paolini S, Pasquarello A, Paulatto L, Sbraccia C, Scandolo S, Sclauzero G, Seitsonen AP, Smogunov A, Umari P, and Wentzcovitch RM (2009) QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys.: Condens. Matter 21:395502
Giannozzi P, Andreussi O, Brumme T, Bunau O, Nardelli MB, Calandra M, Car R, Cavazzoni C, Ceresoli D, Cococcioni M, Colonna N, Carnimeo I, Dal Corso A, de Gironcoli S, Delugas P, DiStasio Jr RA, Ferretti A, Floris A, Fratesi G, Fugallo G, Gebauer R, Gerstmann U, Giustino F, Gorni T, Jia J, Kawamura M, Ko H-Y, Kokalj A, Küçükbenli E, Lazzeri M, Marsili M, Marzari N, Mauri F, Nguyen NL, Nguyen H-V, Otero-de-la-Roza A, Paulatto L, Poncé S, Rocca D, Sabatini R, Santra B, Schlipf M, Seitsonen AP, Smogunov A, Timrov I, Thonhauser T, Umari P, Vast N, Wu X, Baroni S (2017) Advanced capabilities for materials modelling with quantum ESPRESSO. J Phys Condens Matter 29, 465901
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Troullier N, Martins JL (1991) Efficient pseudopotentials for plane-wave calculations. Phys Rev B 43:1993–2006
Ortega-Castro J, Hernández-Haro N, Hernández-Laguna A, Sainz-Díaz CI (2008) DFT calculation of crystallographic properties of dioctahedral 2:1 phyllosilicates. Clay Miner 43:351–361
Ortega-Castro J, Hernández-Haro N, Muñoz-Santiburcio D, Hernández-Laguna A, Sainz-Díaz CI (2009) Crystal structure and hydroxyl group vibrational frequencies of phyllosilicates by DFT methods. J Mol Struct THEOCHEM 912:82–87. https://doi.org/10.1016/j.theochem.2009.02.013
Ceperley DM, Alder BJ (1980) Ground state of the electron gas by a stochastic method. Phys Rev Lett 45:566–569
White CE, Provis JL, Riley DP, Kearley GJ, van Deventer JSJ (2009) What is the structure of kaolinite? Reconciling theory and experiment. J Phys Chem B 113:6756–6765
Tunega D, Bučo T, Zaoui A (2012) Assessment of ten DFT methods in predicting structures of sheet silicates: importance of dispersion corrections. J Chem Phys 137:114105
Blöchl PE (1994) Projector augmented-wave method. Phys Rev B 50:17953–17979
Becke AD (1986) On the large-gradient behavior of the density functional exchange energy. J Chem Phys 85:7184
Becke AD, Johnson ER (2007) Exchange-hole dipole moment and the dispersion interaction revisited. J Chem Phys 127:154108
de-la Roza AO, Johnson ER (2012) Van der Waals interactions in solids using the exchange-hole dipole moment model. J Chem Phys 136:174109
Dal Corso A., Pseudopotential Periodic Table: From H to Pu (2014) Comput Mater Sci 95, 337
Angel RJ (2000) Equations of state. In: Hazen RM, Downs RT (eds) High-pressure and high-temperature crystal chemistry Review in Mineralogy and Geochemistry, vol 41, pp 35–60
Angel RJ, Gonzalez-Platas J, Alvaro M (2014) Eosfit7c and a Fortran module (library) for equation of state calculations (2014) Z. Kristallogr. 229(5): 405–419. http://www.ccp14.ac.uk/ccp/web-mirrors/ross-angel/rja/soft/
Guggenheim EA (1937) Theoretical basis of Raoult’s law. Trans Faraday Soc 33:151–159
Redlich O, Kister T (1948) Algebraic representation of thermodynamic properties and the classification of solutions. Ind Eng Chem 40:345–348
Ganguly J (2008) Thermodynamics in earth and planetary sciences. Springer, Heidelberg
Roux J, Hovis GL (1996) Thermodynamic mixing model for muscovite-paragonite solutions based on solutions calorimetric and phase equilibrium data. J Petrol 57:1241–1254
Slaughter M (1966) Chemical binding in silicate minerals. Geochim Cosmochim Acta 30:299–339
Yu J-Y (1994) Theoretical calculation of Gibbs free energy of mixing between phlogopite and eastonite. J Geol Soc Korea 30:578–590
Yu J-Y (1997) Theoretical calculation of Gibbs free energy of mixing biotite: phlogopite-annite-eastonite-siderophyllite system. Geosci J 1:179–188
Helgeson HC, Delany JM, Nesbitt HW, Bird DK (1978) Summary and critique thermodynamic properties of rock forming minerals. Am J Sci 278A:1–229
Holland TJB, Powell R (2011) An improved and extended internally consistent thermodynamic dataset for phases of petrological interest, involving a new equation of state for solids. J Metamorph Geol 29:333–383
Price JG (1985) Ideal site mixing in solid solutions, with an application to two-feldspar geothermometry. Am Mineral 70:696–701
Nordstrom DK, Munoz JL (1985) Geochemical thermodinamycs. Benjamin /Cummings Publishing Co. Inc., Menlo Park 0-8053-6816-7
Pavese A, Levy D, Curetti N, Diella V, Fumagalli P, Sani A (2003) Equation of state and compressibility of phlogopite by in-situ high-pressure X-ray powder diffraction. Eur J Mineral 15:455–463
Comodi P, Fumagalli P, Montagnoli M, Zanazzi PF (2004) A single-crystal study on the pressure behavior of phlogopite and petrological implications. Am Mineral 89:647–653
Scordari F, Schingaro E, Mesto E, Lacalamita M (2012) 2M 1 –phlogopite from Black Hills (South Australia): the first case of configurational polytype in micas. Am Mineral 97:2016–2023
Burnham CW, Radoslovich EW (1964) Crystal structure of coexisting muscovite and paragonite. Carnegie Inst Wash Year Books 63:232–236
Rothbauer R (1971) Untersuchung eines 2M1-muskovits mit neutronenstrahlen. Neues Jb Mineral Monat 1971:143–154
Guggenheim S, Chang Y-H, Koster van Groos AF (1987) Muscovite dehydroxylation: high-temperature studies. Am Mineral 72:537–550
Catti M, Ferraris G, Ivaldi G (1989) Thermal strain analysis in the crystal structure of muscovite 2M1 at 700 °C. Eur J Mineral 1:625–632
Catti M, Ferraris G, Hull S, Pavese A (1994) Powder neutron diffraction study of 2M1 muscovite at room pressure and at 2 GPa. Eur J Mineral 6:171–178
Guidotti CV, Mazzoli C, Sassi FP, Blencoe JG (1992) Compositional controls on the cell dimensions of 2M1 muscovite and paragonite. Eur J Mineral 4:283–297
Brigatti MF, Frigieri P, Poppi L (1998) Crystal chemistry of Mg-, Fe-bearing muscovites-2M 1. Am Mineral 83:775–785
Mookherjee M, Redfern SAT, Zhang M (2001) Thermal response of structure and hydroxyl ion of phengite-2M 1: an in situ, neutron diffraction and FTIRstudy. Eur J Mineral 13:545–555
Mookherjee M, Redfern SAT (2002) A high-temperature Fourier transform infrared study of the interlayer and Si-O-stretching region in phengite-2M 1. Clay Miner 37:323–336
Comodi P, Zanazzi PF (1995) High-pressure structural study of muscovite. Phys Chem Miner 22:170–177
Comodi P, Zanazzi PF (1997) Pressure dependence of structural parameters of paragonite. Phys Chem Miner 24:274–280
Vaughan MT, Guggenheim S (1986) Elasticity of muscovite and its relationship to crystal structure. J Geophys Res 91:4657–4664
Anderson OL (1995) Equation of state of solids for geophysical and ceramic science. Oxford University Press, Oxford monographs on geology and geophysics 0-19-505606-X
Ferraris C, Grobety B, Weissicken R (2001) Phlogopite exsolutions within muscovite: a first evidence for a higher-temperature re-equilibration, studied by HRTEM and AEM techniques. Eur J Mineral 13:15–26
Molina-Montes E, Donadio D, Hernández-Laguna A, Sainz-Díaz CI, Parrinello M (2008a) DFT research on the dehydroxylation reaction of Pyrophyllite. 1. First principle molecular dynamics simulations. J Phys Chem B 112:7051–7060
Molina-Montes E, Donadio D, Hernández-Laguna A, Sainz-Díaz I (2008b) DFT research on the dehydroxylation reaction of Pyrophyllite. 2. Characterization of reactants, intermediates and transition states along the reaction path. J Chem Phys A 112:6373–7383
Molina-Montes E, Timón V, Hernández-Laguna A, Sainz-Díaz CI (2008c) Dehydroxylation mechanisms in Al3+/Fe3+ dioctahedral phyllosilicates by quantum mechanical methods with cluster models. Geochim Cosmochim Acta 72:3929–3938
Molina-Montes E, Donadio D, Hernández-Laguna A, Parrinello M, Sainz-Díaz CI (2013) Water release from pyrophyllite during the dehydroxylation process explored by quantum mechanical simulations. J Phys Chem C 117:7526–7532
Muñoz-Santiburcio D, Kosa M, Hernández-Laguna A, Sainz-Díaz CI, Parrinello M (2012) Ab initio molecular dynamics study of the Dehydroxylation Reaction in a Smectite model. J Phys Chem C 116:12203–12211
Muñoz-Santiburcio D, Hernández-Laguna A, Sainz-Díaz CI (2016) Simulating the Dehydroxylation reaction in Smectite models by Car−Parrinello-like−born−Oppenheimer molecular dynamics and Metadynamics. J Phys Chem C 120:28186–28192
Acknowledgements
The authors thank the “Centro de Supercomputación de Galicia” (CESGA) and “Centro de Servicios de Informática y Redes de Comunicaciones (CSIRC), Universidad de Granada” for providing the computing time. The authors are thankful to F. Muñoz-Izquierdo, J. Rodríguez-Fernández, and M. Mookherjee for fruitful suggestions. This work was supported by Spanish MCINN and European FEDER grants CGL2008-02850/BTE, FIS2013-48444-C2-2P, FIS2016-77692-C2-2P, and PCIN-2017-098 and by the regional agency “Junta de Andalucía” for the RNM-264, -363, and -1897 PAI-grants.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper belongs to Topical Collection QUITEL 2018 (44th Congress of Theoretical Chemists of Latin Expression)
Electronic supplementary material
ESM 1
(DOCX 32 kb)
Rights and permissions
About this article
Cite this article
Hernández-Laguna, A., Pérez del Valle, C., Hernández-Haro, N. et al. Compressibility of 2M1 muscovite-phlogopite series minerals. J Mol Model 25, 341 (2019). https://doi.org/10.1007/s00894-019-4218-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4218-x