Abstract
In this work, we investigate the adsorption process of CO2 in graphene quantum dots from the electronic structure and spectroscopic properties point of view. We discuss how a specific doping scheme could be employed to further enhance the adsorbing properties of the quantum dots. This is evaluated by considering the depth of the potential well, the spectroscopic constants, and the lifetime of the compound. Electronic structure calculations are carried out in the scope of the density functional theory (DFT), whereas discrete variable representation (DVR) and Dunham methodologies are employed to obtain spectroscopic constants and hence the lifetimes of the systems. Our results suggest that nitrogen-doped graphene quantum dots are promising structures as far as sensing applications of CO2 are concerned.

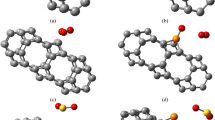
Adsorption mechanism of the CO2 molecule in (a) a pristine and (b) a nitrogendoped Graphene Quantum Dot



Similar content being viewed by others
References
Ren W, Cheng H (2014) . Nat Nanotechnol 9(10):726
da Cunha WF, Ribeiro LA Jr, Fonseca ALDA, Gargano R, e Silva GM (2014) . J Phys Chem C 118(41):23451
de Oliveira Neto PH, Van Voorhis T (2018) . Carbon 132:352
Paura ENC, da Cunha WF, de Oliveira Neto PH, e Silva GM, Martins JB, Gargano R (2013) . J Phys Chem A 117(13):2854
Manzoli A, Steffens C, Paschoalin RT, Correa AA, Alves WF, Leite FL, Herrmann PS (2011) . Sensors 11(6):6425
Raeyani D, Shojaei S, Kandjani SA, Wlodarski W (2016) . Procedia Eng 168:1312
Lin YC, Lin CY, Chiu PW (2010) . Appl Phys Lett 96(13):133110
Liu H, Liu Y, Zhu D (2011) . J Mater Chem 21(10):3335
Ma L, Hu H, Zhu L, Wang J (2011) . J Phys Chem C 115(14):6195
Gong K, Du F, Xia Z, Durstock M, Dai L (2009) . Science 323(5915):760
Li Y, Zhao Y, Cheng H, Hu Y, Shi G, Dai L, Qu L (2011) . J Am Chem Soc 134(1):15
Wolfgang R (1970) . Acc Chem Res 3(2):48
Pitsevich G, Malevich A (2016) . J Appl Spectrosc 82(6):893
Light J, Hamilton I, Lill J (1985) . J Chem Phys 82(3):1400
Dunham J (1932) . Phys Rev 41(6):721
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 16 Revision B.01. Gaussian Inc., Wallingford
Maniero AM, Acioli PH (2005) . Int J Quantum Chem 103(5):711
Powell M (1965) . Comput J 7(4):303
da Cunha WF, de Oliveira RM, Roncaratti LF, Martins JB, e Silva GM, R Gargano (2014) . J Mol Model 20(12):2498
Slater N (1939) .. In: Mathematical proceedings of the Cambridge Philosophical Society, vol 35, vol 35. Cambridge University Press, Cambridge, pp 56–69
Acknowledgements
The authors acknowledge the financial support from Brazilian agencies CNPq and FAP-DF. P.H.O.N. and W.F.C. also acknowledge the financial support from FAP-DF grants 0193.001662/2017, and 0193.001694/2017 respectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper belongs to the Topical Collection VII Symposium on Electronic Structure and Molecular Dynamics – VII SeedMol
Rights and permissions
About this article
Cite this article
de Oliveira Neto, P.H., Rodrigues, J.P.C.C., de Sousa, L.E. et al. CO2 adsorption in nitrogen-doped single-layered graphene quantum dots: a spectroscopic investigation. J Mol Model 25, 66 (2019). https://doi.org/10.1007/s00894-019-3951-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-3951-5