Abstract
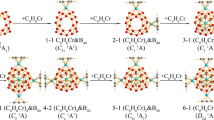
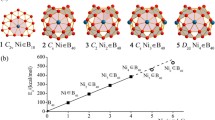
Neutral closo-dicarboboranes are reported to have very low H2 binding ability. Herein, we report an improvement in H2 binding energy (Eb) of C2B4H6 by substituting H atoms with different functional groups like X = F, Cl, Br, and XY = BO, CN and NC via quantum-chemical density functional theory based computations. In going from B6H62− to C2B4H6, the Eb value is reduced from 14.6 kJ mol−1 to 2.7 kJ mol−1. C2B4X6 and C2B4(XY)6 systems, which can bind a total of eight H2 molecules, with one H2 molecule occupying at each B-B-C face, possess an Eb value per H2 in the range of 4.5 kJ mol−1 for X = F, 3.9 kJ mol−1 for X = Cl, 5.9 kJ mol−1 for X = Br, 6.8 kJ mol−1 for XY = BO, 5.8 kJ mol−1 for XY = CN and 5.2 kJ mol−1 for XY = NC. The improvement in Eb value is found to be the highest in case of C2B4(BO)6, which has the ability to bind 6.6 gravimetric wt% of H2. The situation can be made more favorable by applying an external electric field. Energy decomposition analysis reveals that although the dispersion interaction (ca. 55–65%) has significant role in binding H2 with such types of molecules, contribution from electrostatic and orbital interaction is also considerable. Further, we modeled an extended system by linking C2B4(BO)n through ‘C ≡ C’ units for H2 storage purpose. The energy difference between the highest occupied and the lowest unoccupied molecular orbitals gradually lessens with the increase in molecular length. Therefore, it can be tuned gradually by controlling the chain length, which may further open up their potency in the field of electronics.

C2B4X6 (X = F, Cl, Br) and C2B4(XY)6 (XY = BO, CN, NC) show enhanced H2 binding ability from C2B4H6. Further, 1D, 2D and 3-D frameworks can be built by joining C2B4(BO)n units via ‘C ≡ C’ linkage.




Similar content being viewed by others
References
Jena P (2011) Materials for hydrogen storage: past, present, and future. J Phys Chem Lett 2:206–211
Veziroglu TN, Barbir F (1992) Hydrogen: the wonder fuel. Int J Hydrog Energy 17:391–404
Lubitz W, Tumas W (2007) Hydrogen: an overview. Chem Rev 107:3900–3903
Florusse LJ, Peters CJ, Schoonman J, Hester KC, Koh CA, Dec SF, Marsh KN, Sloan ED (2004) Stable low-pressure hydrogen clusters stored in a binary clathrate hydrate. Science 306:469–471
Lee H, Lee J, Kim DY, Park J, Seo YT, Zeng H, Moudrakovski IL, Ratcliffe CI, Ripmeester JA (2005) Tuning clathrate hydrates for hydrogen storage. Nature 434:743–746
McKeown NB, Gahnem B, Msayib KJ, Budd PM, Tattershall CE, Mahmood K, Tan S, Book D, Langmi HW, Walton A (2006). Angew Chem Int Ed 45:1804
Baldé CP, Hereijgers BPC, Bitter JH, de Jong KP (2006) Facilitated hydrogen storage in NaAlH4 supported on carbon nanofibers. Angew Chem Int Ed 45:3501–3503
Rosi NL, Eckert J, Eddaoudi M, Vodak DT, Kim J, O’keeffe M, Yaghi OM (2003) Hydrogen storage in microporous metal-organic frameworks. Science 300:1127–1129
Rowsel JLC, Yaghi OM (2005) Strategies for hydrogen storage in metal–organic frameworks. Angew Chem Int Ed 44:4670–4679
Rowsel JLC, Yaghi OM (2006) Effects of functionalization, catenation, and variation of the metal oxide and organic linking units on the low-pressure hydrogen adsorption properties of metal−organic frameworks. J Am Chem Soc 128:1304–1315
Kuc A, Zhechkov L, Patchkovskii S, Seifert G, Heine T (2007) Hydrogen sieving and storage in fullerene intercalated graphite. Nano Lett 7:1–5
Han SS, Furukawa H, Yaghi OM, Goddard III WA (2008) Covalent organic frameworks as exceptional hydrogen storage materials. J Am Chem Soc 130:11580–11581
Pan S, Giri S, Chattaraj PK (2012) A computational study on the hydrogen adsorption capacity of various lithium—doped boron hydrides. J Comp Chem 33:425–434
Blomqvist A, Araújo CM, Srepusharawoot P, Ahuja R (2007) Li-decorated metal–organic framework 5: a route to achieving a suitable hydrogen storage medium. Proc Natl Acad Sci USA 104:20173–20176
Pan S, Merino G, Chattaraj PK (2012) The hydrogen trapping potential of some li-doped star-like clusters and super-alkali systems. Phys Chem Chem Phys 14:10345–10350
Deng WQ, Xu X, Goddard WA (2004) New alkali doped pillared carbon materials designed to achieve practical reversible hydrogen storage for transportation. Phys Rev Lett 92:166103
Mpourmpakis G, Froudakis GE (2006) SiC nanotubes: a novel material for hydrogen storage. Nano Lett 6:1581–1583
Heine T, Zhechkov L, Seifert G (2004) Hydrogen storage by physisorption on nanostructured graphite platelets. Phys Chem Chem Phys 6:980–984
Wu X, Gao Y, Zeng XC (2008) Hydrogen storage in pillared li-dispersed boron carbide nanotubes. J Phys Chem C 112:8458–8463
Sun Q, Wang Q, Jena P (2005) Storage of molecular hydrogen in B−N cage: energetics and thermal stability. Nano Lett 5:1273–1277
Kojima Y, Suzuki KI, Fukumoto K, Sasaki M, Yamamoto T, Kawai Y, Hayashi H (2002) Hydrogen generation using sodium borohydride solution and metal catalyst coated on metal oxide. Int J Hydrog Energy 27:1029–1034
Pan S, Mondal S, Chattaraj PK (2013) Cucurbiturils as promising hydrogen storage materials: a case study of cucurbit [7] uril. New J Chem 37:2492–2499
Pan S, Banerjee S, Chattaraj PK (2012) Role of lithium decoration on hydrogen storage potential. J Mex Chem Soc 56:229–240
Dienberg L, Haug J, Rauhut G, Roduner E (2013) Hydrogen storage by physisorption on dodecahydro-closo-dodecaboranes. Phys Chem Chem Phys 15:5836–5843
Srinivasu K, Ghosh SK (2011) An ab initio investigation of hydrogen adsorption in li-doped closo-boranes. J Phys Chem C 115:1450–1456
Lu QL, Meng JW, Song WJ, Wan JG (2013) High capacity hydrogen storage in closo-hexaborate dianion B6H6 2−. Int J Hydrog Energy 38:13328–13334
Pathak B, Pradhan K, Hussain T, Ahuja R, Jena P (2012) Functionalized boranes for hydrogen storage. Chem Phys Chem 13:300–304
Lu QL, Huang SG, Li YD (2013) Hydrogen storage of mg-decorated closo-hexaborate B6H6 2−. Acta Phys Sin 62:213601
Zhang CG, Zhang R, Wang ZX, Zhou Z, Zhang SB, Chen Z (2009) Ti-substituted boranes as hydrogen storage materials: a computational quest for the ideal combination of stable electronic structure and optimal hydrogen uptake. Chem Eur J 15:5910–5919
Gopalsamy K, Prakash M, Kumar RM, Subramanian V (2012) Density functional studies on the hydrogen storage capacity of boranes and alanes based cages. Int J Hydrog Energy 37:9730–9741
Nam W, Onak T (1987) Relative Reactivities of the small Closo Carboranes 1,6-C2B4H6 and 2,4-C2B5H7 and of closo-l,10-C2B8H10 toward "electrophilic" reagents. Inorg Chem 26:1581–1586
Zint N, Dreuw A, Cederbaum LS (2002) Gas-phase stability of derivatives of the closo-Hexaborate Dianion B6H6 2−. J Am Chem Soc 124:4910–4917
Dreuw A, Zint N, Cederbaum LS (2002) Dianionic Tetraborates do exist as stable entities. J Am Chem Soc 124:10903–10910
Preetz W, Fritze J, Darstellung Z (1984) 11B-NMR- und Schwingungsspektren der oktaedrischen closo-Boratanionen B6X6 2−; X = H, Cl, Br, I. Z Naturforsch B 39:1472
Preetz W, Peters G (1999) The hexahydro-closo-hexaborate dianion [B6H6]2− and its derivatives. Eur J Inorg Chem 1999:1831–1846
Thomsen H, Haeckel O, Krause U, Preetz W (1996) Darstellung und spektroskopische charakterisierung der monofluorohydro-closo-borate [B6H5F]2− und [B12H11F]2−. Z Anorg Allg Chem 622:2061
Preetz W, Franken A, Rath M, Preetz W, Franken A, Rath M, Darstellung Z (1993) 11B-, 13C-NMR- und Schwingungsspektren der closo-Hexaborate [B6H5(CN)]2− und cis-[B6H4(CN)2]2− sowie Kristallstruktur von Cs2[B6H5(CN)] / preparation, 11B,13C NMR and vibrational spectra of the closo-Hexaborates [B6H5(CN)]2- and cis-[B6H4(CN)2]2-, and the crystal structure of Cs2[B6H5(CN)]. Z Naturforsch B 48:598–602
Ziegler T, Rauk A (1977) On the calculation of bonding energies by the Hartree Fock slater method. Theor Chim Acta 46:1–10
Zhao Y, Schultz NE, Truhlar DG (2006) Design of Density Functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J Chem Theory Comput 2:364–382
Ditchfield R, Hehre WJ, Pople JA (1971) Self-consistent molecular-orbital methods. IX. an extended gaussian-type basis for molecular-orbital studies of organic molecules. J Chem Phys 54:724–728
Frisch MJ et al (2009) Gaussian 09, revision a.1. Gaussian, Inc, Wallingford
Burns LA, Vázquez-Mayagoitia A, Sumpter BG, Sherrill CD (2011) Density-functional approaches to noncovalent interactions: a comparison of dispersion corrections (DFT-D), exchange-hole dipole moment (XDM) theory, and specialized functional. J Chem Phys 134:084107
Martínez SH, Pan S, Cabellos JL, Dzib E, Fernández-Herrera MA, Merino G (2017) Importance of dispersion on the stability of the concave-bound CpM (M = Fe, Ru, Os) complexes of Sumanene. Organometallics 36:2036–2041
ADF2017, SCM Theoretical chemistry. Vrije Universiteit, Amsterdam, The Netherlands http://www.scm.com
te Velde G, Bickelhaupt FM, Baerends EJ, Guerra CF, Van Gisbergen SJA, Snijders JG, Ziegler T (2001) Chemistry with ADF. J Comput Chem 22:931–967
Pan S, Saha R, Mandal S, Chattaraj PK (2016) σ-Aromatic cyclic M3 + (M = cu, ag, au) clusters and their complexation with dimethyl imidazol-2-ylidene, pyridine, isoxazole, furan, noble gases and carbon monoxide. Phys Chem Chem Phys 18:11661–11676
Pan S, Saha R, Chattaraj PK (2015) Exploring the nature of silicon-noble gas bonds in H3SiNgNSi and HSiNgNSi compounds (ng = Xe, Rn). Int J Mol Sci 16:6402–6418
Pan S, Moreno D, Merino G, Chattaraj PK (2014) Stability of Noble gas bound SiH3 + clusters. Chem Phys Chem 15:3554–3564
Pan S, Moreno D, Cabellos JL, Merino G, Chattaraj PK (2014) Ab initio study on the stability of NgnBe2N2, NgnBe3N2 and NgBeSiN2 clusters. Chem Phys Chem 15:2618–2625
Khatua M, Pan S, Chattaraj PK (2014) Movement of Ng2 molecules confined in a C60 cage: an ab initio molecular dynamics study. Chem Phys Lett 610-611:351–356
Pan S, Jalife S, Romero J, Reyes A, Merino G, Chattaraj PK (2013) Attractive Xe–li interaction in li-decorated clusters. Comput Theor Chem 1021:62–69
Liu L, Moreno D, Osorio E, Castro AC, Pan S, Chattaraj PK, Heine T, Merino G (2016) Structure and bonding of IrB12 −: converting a rigid boron B12 platelet to a Wankel motor. RSC Adv 6:27177–27182
Vargas-Caamal A, Pan S, Ortiz-Chi F, Cabellos JL, Boto RA, Contreras-Garcia J, Restrepo A, Chattaraj PK, Merino G (2016) How strong are the metallocene–metallocene interactions? Cases of ferrocene, ruthenocene, and osmocene. Phys Chem Chem Phys 18:550–556
Zhao L, von Hopffgarten M, Andrada DM, Frenking G (2018) Energy decomposition analysis. WIREs Comput Mol Sci 8:e1345
Frenking G, Bickelhaupt FM (2014) The EDA perspective of chemical bonding. In: Frenking G, Shaik S (eds) The chemical bond 1. Fundamental aspects of chemical bonding. Wiley-VCH, Weinheim, pp 121–158
Ramsey NF (1950) Quadrupole moment of the Electron distribution in hydrogen molecules. Phys Rev 78:221–222
Pearson RG (1993) The principle of maximum hardness. Acc Chem Res 26:250–255
Pan S, Solà M, Chattaraj PK (2013) On the validity of the maximum hardness principle and the minimum electrophilicity principle during chemical reactions. J Chem Phys A 117:1843–1852
Zhou J, Wang Q, Sun Q, Jena P, Chen XS (2010) Electric field enhanced hydrogen storage on polarizable materials substrates. Proc Natl Acad Sci USA 107:2801–2806
Sun X, Hwang JY, Shi S (2010) Hydrogen Storage in Mesoporous Metal Oxides with Catalyst and External Electric Field. J Phys Chem C 114:7178–7184
Pan S, Contreras M, Romero J, Reyes A, Chattaraj PK, Merino G (2013) C5Li7 + and O2Li5 + as noble gas trapping agents. Chem Eur J 19:2322–2329
Yoshikawa A (2007) Development and applications of wide bandgap semiconductors. In: Yoshikawa A, Matsunami H, Nanishi Y (eds) Wide bandgap semiconductors. Springer, Berlin, pp 1–24
Acknowledgments
SP thanks Nanjing Tech University for the postdoctoral fellowship and the High Performance Computing Center of Nanjing Tech University for supporting the computational resources. LZ acknowledges financial support from the Natural Science Foundation of Jiangsu Province (Grant no: BK20170964), the National Natural Science Foundation of China (grant no. 21703099), and a SICAM Fellowship from Jiangsu National Synergetic Innovation Center for Advanced Materials. The work in Mexico was supported by Conacyt (grant CB-2015-252356).
Author information
Authors and Affiliations
Corresponding authors
Additional information
This paper belongs to Topical Collection International Conference on Systems and Processes in Physics, Chemistry and Biology (ICSPPCB-2018) in honor of Professor Pratim K. Chattaraj on his sixtieth birthday
Electronic supplementary material
ESM 1
(DOCX 3083 kb)
Rights and permissions
About this article
Cite this article
Pan, S., Zhao, L. & Merino, G. Improvement in hydrogen binding ability of closo-dicarboranes via functionalization and designing of extended frameworks. J Mol Model 24, 307 (2018). https://doi.org/10.1007/s00894-018-3827-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3827-0