Abstract
Objective
To assess the efficacy of three mechanical decontamination methods in four types of commercially available implants.
Material and methods
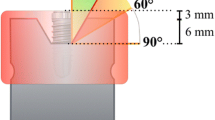
Ninety-six implants of four commercial brands with different designs (regarding thread depth and thread pitch) were soaked in a surrogate biofilm (ink) and air-dried. Circumferential standardized peri-implant defects with 6 mm in depth and 1.55 mm in width were custom-made with a 3D printer. Stained implants were inserted in the defects and instrumented with three different methods: a titanium brush (TNB), a metallic ultrasonic tip (IST) and an air abrasive (PF). Standardized photographs were taken vertically to the implant axis (flat view), and with angulations of 60° (upper view) and 120° (lower view) to the implant long axis. The percentage of residual stain (PRS) was calculated with the image analysis software. Scanning electron microscope evaluations were performed on the buccal aspect of the implants at the central level of the defect.
Results
The efficacy of PF was significantly inferior to the TNB and IST in all implant designs, while there were no significant differences between TNB and IST. IST showed significantly higher PRS in the implant with the highest thread pitch, while the TNB had the highest PRS in the implant with a marked reverse buttress-thread design. The micro-thread design had the lowest values of PRS for all decontamination methods. The apically facing threads represented the areas with highest PRS for all implant designs and decontamination methods.
Conclusion
Thread geometry influenced the access of the decontamination devices and in turn its efficacy. Implants with lower thread pitch and thread depth values appeared to have less residual staining.
Clinical relevance
Clinicians must be aware of the importance of thread geometry in the decontamination efficacy.






Similar content being viewed by others
References
Berglundh T, Armitage G, Araujo MG, Avila-Ortiz G, Blanco J, Camargo PM, Chen S, Cochran D, Derks J, Figuero E, Hammerle CHF, Heitz-Mayfield LJA, Huynh-Ba G, Iacono V, Koo KT, Lambert F, McCauley L, Quirynen M, Renvert S, Salvi GE, Schwarz F, Tarnow D, Tomasi C, Wang HL, Zitzmann N (2018) Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol 89(Suppl 1):S313–s318
Derks J, Tomasi C (2015) Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol 42(Suppl 16):S158–S171
Tomasi C, Regidor E, Ortiz-Vigon A, Derks J (2019) Efficacy of reconstructive surgical therapy at peri-implantitis-related bone defects. A systematic review and meta-analysis. J Clin Periodontol 46(Suppl 21):340–356
Faggion CM Jr, Chambrone L, Listl S, Tu YK (2013) Network meta-analysis for evaluating interventions in implant dentistry: the case of peri-implantitis treatment. Clin Implant Dent Relat Res 15(4):576–588
Carcuac O, Derks J, Abrahamsson I, Wennstrom JL, Petzold M, Berglundh T (2017) Surgical treatment of peri-implantitis: 3-year results from a randomized controlled clinical trial. J Clin Periodontol 44(12):1294–1303
Persson LG, Araujo MG, Berglundh T, Grondahl K, Lindhe J (1999) Resolution of peri-implantitis following treatment. An experimental study in the dog. Clin Oral Implants Res 10(3):195–203
Roccuzzo M, Pittoni D, Roccuzzo A, Charrier L, Dalmasso P (2017) Surgical treatment of peri-implantitis intrabony lesions by means of deproteinized bovine bone mineral with 10% collagen: 7-year-results. Clin Oral Implants Res 28(12):1577–1583
Schwarz F, Jepsen S, Herten M, Sager M, Rothamel D, Becker J (2006) Influence of different treatment approaches on non-submerged and submerged healing of ligature induced peri-implantitis lesions: an experimental study in dogs. J Clin Periodontol 33(8):584–595
Schwarz F, Claus C, Becker K (2017) Correlation between horizontal mucosal thickness and probing depths at healthy and diseased implant sites. Clin Oral Implants Res 28(9):1158–1163
Persson LG, Ericsson I, Berglundh T, Lindhe J (2001) Osseintegration following treatment of peri-implantitis and replacement of implant components. An experimental study in the dog. J Clin Periodontol 28(3):258–263
Schwarz F, Sculean A, Bieling K, Ferrari D, Rothamel D, Becker J (2008) Two-year clinical results following treatment of peri-implantitis lesions using a nanocrystalline hydroxyapatite or a natural bone mineral in combination with a collagen membrane. J Clin Periodontol 35(1):80–87
Schwarz F, Hegewald A, Sahm N, Becker J (2014) Long-term follow-up of simultaneous guided bone regeneration using native and cross-linked collagen membranes over 6 years. Clin Oral Implants Res 25(9):1010–1015
Roccuzzo M, Gaudioso L, Lungo M, Dalmasso P (2016) Surgical therapy of single peri-implantitis intrabony defects, by means of deproteinized bovine bone mineral with 10% collagen. J Clin Periodontol 43(3):311–318
Froum SJ, Froum SH, Rosen PS (2015) A regenerative approach to the successful treatment of peri-implantitis: a consecutive series of 170 implants in 100 patients with 2- to 10-year follow-up. Int J Periodontics Restorative Dent 35(6):857–863
Deppe H, Horch HH, Neff A (2007) Conventional versus CO2 laser-assisted treatment of peri-implant defects with the concomitant use of pure-phase beta-tricalcium phosphate: a 5-year clinical report. Int J Oral Maxillofac Implants 22(1):79–86
Sahrmann P, Ronay V, Sener B, Jung RE, Attin T, Schmidlin PR (2013) Cleaning potential of glycine air-flow application in an in vitro peri-implantitis model. Clin Oral Implants Res 24(6):666–670
Sahrmann P, Ronay V, Hofer D, Attin T, Jung RE, Schmidlin PR (2015) In vitro cleaning potential of three different implant debridement methods. Clin Oral Implants Res 26(3):314–319
Ronay V, Merlini A, Attin T, Schmidlin PR, Sahrmann P (2017) In vitro cleaning potential of three implant debridement methods. Simulation of the non-surgical approach. Clin Oral Implants Res 28(2):151–155
Charalampakis G, Ramberg P, Dahlen G, Berglundh T, Abrahamsson I (2015) Effect of cleansing of biofilm formed on titanium discs. Clin Oral Implants Res 26(8):931–936
Steiger-Ronay V, Merlini A, Wiedemeier DB, Schmidlin PR, Attin T, Sahrmann P (2017) Location of unaccessible implant surface areas during debridement in simulated peri-implantitis therapy. BMC Oral Health 17(1):137
Polak D, Maayan E, Chackartchi T (2017) The impact of implant design, defect size, and type of superstructure on the accessibility of nonsurgical and surgical approaches for the treatment of peri-implantitis. Int J Oral Maxillofac Implants 32(2):356–362
Abuhussein H, Pagni G, Rebaudi A, Wang HL (2010) The effect of thread pattern upon implant osseointegration. Clin Oral Implants Res 21(2):129–136
Unursaikhan O, Lee JS, Cha JK, Park JC, Jung UW, Kim CS, Cho KS, Choi SH (2012) Comparative evaluation of roughness of titanium surfaces treated by different hygiene instruments. J Periodontal Implant Sci 42(3):88–94
Keim D, Nickles K, Dannewitz B, Ratka C, Eickholz P, Petsos H (2019) In vitro efficacy of three different implant surface decontamination methods in three different defect configurations. Clin Oral Implants Res 30(6):550–558
Laleman I, Cortellini S, De Winter S, Rodriguez Herrero E, Dekeyser C, Quirynen M, Teughels W (2017) Subgingival debridement: end point, methods and how often? Periodontol 75(1):189–204
Cha JK, Paeng K, Jung UW, Choi SH, Sanz M, Sanz-Martin I (2019) The effect of five mechanical instrumentation protocols on implant surface topography and roughness: a scanning electron microscope and confocal laser scanning microscope analysis. Clin Oral Implants Res 30(6):578–587
Mengel R, Buns CE, Mengel C, Flores-de-Jacoby L (1998) An in vitro study of the treatment of implant surfaces with different instruments. Int J Oral Maxillofac Implants 13(1):91–96
Mann M, Parmar D, Walmsley AD, Lea SC (2012) Effect of plastic-covered ultrasonic scalers on titanium implant surfaces. Clin Oral Implants Res 23(1):76–82
Ruhling A, Kocher T, Kreusch J, Plagmann HC (1994) Treatment of subgingival implant surfaces with Teflon-coated sonic and ultrasonic scaler tips and various implant curettes. An in vitro study. Clin Oral Implants Res 5(1):19–29
Boggan RS, Strong JT, Misch CE, Bidez MW (1999) Influence of hex geometry and prosthetic table width on static and fatigue strength of dental implants. J Prosthet Dent 82(4):436–440
Acknowledgements
The authors would like to acknowledge the support of Prof. Ji-Man Park with the 3D printed models.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2019R1C1C1006622).
Author information
Authors and Affiliations
Contributions
Ignacio Sanz-Martín: conception, design of the work, conduct of the experiment, data analysis and drafting the manuscript.
Kyeongwon Paeng: conduct of the experiment and data analysis.
Hyobin Park: conduct of the experiment and data analysis.
Jae-Kook Cha: conception, design of the work, conduct of the experiment, data analysis and support in manuscript writing.
Ui-Won Jung: critical revision of manuscript.
Mariano Sanz: critical revision of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Summary: Thread geometry influenced the access of the decontamination devices and in turn its efficacy.
Supplementary information
ESM 1
(DOCX 172 kb)
Rights and permissions
About this article
Cite this article
Sanz-Martín, I., Paeng, K., Park, H. et al. Significance of implant design on the efficacy of different peri-implantitis decontamination protocols. Clin Oral Invest 25, 3589–3597 (2021). https://doi.org/10.1007/s00784-020-03681-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03681-y