Abstract
Objectives
The aim of this study was to analyze the influence of dietary fatty acids (FAs) and the time elapsed from their intake on FA tissue profile of rat submandibular gland (SG) and on its salivary flow rate (SFR). Do dietary FAs depending on the intake time modify their profile in SG and consequently the SFR?
Materials and methods
Thirty-six adult male Wistar rats were fed on control diet (corn oil, CD, 18:2 n-6 FA) for 7 days and then divided into CD and two groups with replacement of corn oil by olive (OD, 18:1 n-9 FA) or chia (ChD, 18:3 n-3 FA) oils (1 and 30 day intake). Submandibular ducts were canalized to collect saliva for 20 min (μL/min). SG were examined (optical/electron microscopy; ImageJ 1.48 software).
Results
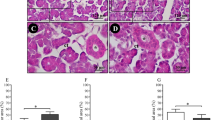
SFR values were 6.18 ± 0.34 (CD1), 6.04 ± 0.31 (OD1), and 6.00 ± 0.50 (ChD1) (p > 0.05). At 30-day intake, higher SFR values in ChD (7.82 ± 0.7) with respect to CD (4.68 ± 0.44; p < 0.001) and OD (6.08 ± 0.2; p = 0.038) were found. ChD30 showed a higher serous acinous area percentage than CD30 and OD30, whereas mucous acinous density was greater in CD30 than in OD30 and ChD30 (p < 0.05). α-Linolenic (ALA) and eicosapentaenoic and docosahexaenoic acid levels were only detected in SG of ChD30, while arachidonic acid was lower in this group as compared with CD30 and OD30 (p < 0.05).
Conclusions
SG FA composition and its SFR appear to be modulated by dietary FAs and the time elapsed from their consumption. SFR is highest with n-3 ALA-rich ChD at 30-day intake.
Clinical relevance
Diet could contribute to improve secretory dysfunctions.



Similar content being viewed by others
References
Dodds W, Johnson D, Yeh C (2005) Health benefits of saliva: a review. J Dent 3:223–233
Koneru S, Tanikonda R (2014) Salivaomics: a promising future in early diagnosis of dental diseases. Dent Res J 11:11–15
Dawes C, Pedersen A, Villa A et al (2015) The functions of human saliva: a review sponsored by the world workshop on oral medicine VI. Arch Oral Biol 60:863–874
Sreebny L (2000) Saliva in health and disease: an appraisal and update. Int Dent J 50:140–161
Mese H, Matsuo R (2007) Salivary secretion, taste and hyposalivation. J Oral Rehabil 34:711–723
Chicharro J, Lucia A, Perez M, Vaquero A, Urena R (1998) Saliva composition and exercise. Sports Med 26:17–27
Humphrey S, Williamson R (2001) A review of saliva: normal composition, flow, and function. J Prosthet Dent 85:162–169
Belstrøm D, Holmstrup P, Bardow A, Kokaras A, Fiehn N, Paster B (2016) Comparative analysis of bacterial profiles in unstimulated and stimulated saliva samples. J Oral Microbiol 8:301–312
Turner R, Sugiya H (2002) Understanding salivary fluid and protein secretion. Oral Dis 8:3–11
Iwasaki M, Yoshihara A, Ito K et al (2015) Hyposalivation and dietary nutrient intake among community-based older Japanese. Geriatr Gerontol Int 1:1–8
Aps J, Martens LC (2005) The physiology of saliva and transfer of drugs into saliva. Forensic Sci Int 10:119–131
Silvestre-Rangil J, Bagán L, Silvestre F, Bagán J (2016) Oral manifestations of rheumatoid arthritis. A cross-sectional study of 73 patients. Clin Oral Investig 20:2575–2580
Maciejczyk M, Matczuk J, Żendzian-Piotrowska M et al (2018) Eight-week consumption of high-sucrose diet has a pro-oxidant effect and alters the function of the salivary glands of rats. Nutrients 10:1530
Lasisi TJ, Shittu ST, Alada AR (2018) Re-establishing normal diet following high fat-diet-induced obesity reverses the altered salivary composition in Wistar rats. J Basic Clin Physiol Pharmacol 30:111–120
Reeves P, Nielsen F, Fahey G (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951
Report of the American Institute of Nutrition ad hoc committee on standards for nutritional studies (1977) J Nutr 107: 1340-8
Schneyer C, Schneyer L (1960) Electrolyte levels of rat salivary secretions in relation to fluid-flow rate. Am J Phys 199:55–58
Ohlin P (1964) Isoprenaline as secretory agent in salivary gland. Acta Univ Lund 17:1–8 section II
Johnson D, Cortez J (1988) Chronic treatment with beta adrenergic agonists and antagonists alters the composition of proteins in rat parotid saliva. J Dent Res 67:1103–1118
Koller M, Maeda N, Purushotham K, Scarpace P, Humphreys-Beher M (1992) A biochemical analysis of parotid and submandibular salivary gland function with age after simultaneous stimulation with pilocarpine and isoproterenol in females fisher 344 rats. Arch Oral Biol 37:219–230
Koller M, Maeda N, Scarpace P, Humphreys-Beher M (2000) Desipramine changes salivary gland function, oral microbiota, and oral health in rats. Eur J Pharmacol 408:91–98
Folch J, Lees M, Stanley G (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–508
Cantellops D, Reid A, Eitenmiller R, Long A (1999) Determination of lipids in infant formula powder by direct extraction methylation of lipids and fatty acid methyl esters (FAME) analysis by gas chromatography. J AOAC Int 82:1128–1139
De Paul A, Attademo A, Carón R et al (2009) Neuropeptide glutamic-isoleucine (NEI) specifically stimulates the secretory activity of gonadotrophs in primary cultures of female rat pituitary cells. Peptides 30:2081–2087
Daniels TE, Cox D, Shiboski CH et al (2011) Associations between salivary gland histopathologic diagnoses and phenotypic features of Sjögren’s syndrome (SS) among 1726 registry participants. Arthritis Rheum 63:2021–2030
Infostat v.p.1 (2005) Grupo InfoStat. Facultad de Ciencias Agropecuarias, Universidad Nacional de Córdoba, Argentina
Alam S, Alam B (1988) In vivo incorporation of n-3 fatty acids into membrane lipids of salivary glands and changes in adenylate cyclase activity. Arch Oral Biol 33:295–299
Delporte C, Malaisse W, Jurysta C, Portois L, Sener A, Carpentier Y (2007) Altered fatty acid pattern of phospholipids and triglycerides in the submandibular gland of omega3-depleted rats. Eur J Oral Sci 115:103–110
Rodriguez-Cruz M, Tovar A, Palacios-González P, Prado M, Torres N (2006) Synthesis of long-chain polyunsaturated fatty acids in lactating mammary gland: role of D5 and D6 desaturases, SREBP-1, PPAR, and PGC-1. J Lipid Res 47:553–560
Ahmad S, Alam S, Alam B (1990) Influence of dietary omega-3 fatty acids on transmembrane signaling in rat submandibular salivary glands. Cell Signal 2:2941–2944
Calderón RO, Glocker M, Eynard AR (1998) Lipid and fatty acid composition of different fractions from rat urinary transitional epithelium. Lipids 33:1017–1024
Grasso E, Calderón R (2009) Urinary bladder membrane permeability differentially induced by membrane lipid composition. Mol Cell Biochem 330:163–165
Murakami M, Shachar-Hill B, Steward M, Hill A (2001) The paracellular component of water flow in the rat submandibular salivary gland. J Physiol 537:899–906
Murakami M, Murdiastuti K, Hosoi K, Hill A (2006) AQP and the control of fluid transport in a salivary gland. J Membr Biol 210:91–103
Hashimoto S, Murakami M (2009) Morphological evidence of paracellular transport in perfused rat submandibular glands. J Med Investig 56:395–397
Kawedia J, Nieman M, Boivin G et al (2007) Interaction between transcellular and paracellular water transport pathways through aquaporin 5 and the tight junction complex. Proc Natl Acad Sci 104:3621–3626
Prestifilippo J, Fernández-Solari J, de la Cal C, Iribarne M, Suburo A, Rettori V (2006) Inhibition of salivary secretion by activation of cannabinoid receptors. Exp Biol Med 231:1421–1429
Bruce J, Shuttleworth T, Giovannucci D, Yule D (2002) Phosphorylation of inositol 1,4,5-trisphosphate receptors in parotid acinar cells. A mechanism for the synergistic effects of cAMP on ca++ signaling. J Biol Chem 277:1340–1348
Fernandez-Solari J, Prestifilippo J, Ossola C, Rettori V, Elverdin J (2010) Participation of the endocannabinoid system in lipopolysaccharide-induced inhibition of salivary secretion. Arch Oral Biol 5:583–590
Kopach O, Vats J, Netsyk O, Voitenko N, Irving A, Fedirko N (2011) Cannabinoid receptors in submandibular acinar cells: functional coupling between saliva fluid and electrolytes secretion and Ca++ signalling. J Cell Sci 125:1884–1895
Prestifilippo J, Medina V, Mohn C, Rodriguez P, Elverdin J, Fernandez-Solari J (2013) Endocannabinoids mediate hyposalivation induced by inflammogens in the submandibular glands and hypothalamus. Arch Oral Biol 58:1251–1259
Oddi S, Fezza F, Pasquariello N (2008) Evidence for the intracellular accumulation of anandamide in adiposomes. Cell Mol Life Sci 65:840–850
Matias I, Carta G, Murru E, Petrosino S, Banni S, Di Marzo V (2008) Effect of polyunsaturated fatty acids on endocannabinoid and N-acyl-ethanolamine levels in mouse adipocytes. Biochim Biophys Acta 1781:52–60
Alvheim A, Malde M, Osei-Hyiaman D et al (2012) Dietary linoleic acid elevates endogenous 2-AG and anandamide and induces obesity. Obesity 20:1984–1994
El-Nozahy A, Ismail M (2013) The response of rat submandibular salivary gland to plant protein diet; biological and histochemical study. Int J Health Sci 7:309–315
Funding
The work was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and the Secretaría de Ciencia y Tecnología, Universidad Nacional de Córdoba, Argentina.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Jorge Dario Escandriolo Nackauzi, Raquel Gallará, Gastón Repossi, Claudio Bernal, and Adriana Actis. The original manuscript was written by Jorge Dario Escandriolo Nackauzi, and all authors worked on it in reviewing and editing, and they read and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
Jorge Dario Escandriolo Nackauzi has received a research grant from “Secretaría de Ciencia y Tecnología”, Universidad Nacional de Córdoba. Gastón Repossi declares that he has no conflict of interest. Claudio Bernal declares that he has no conflict of interest. Adriana Actis declares that he has no conflict of interest. Raquel Gallará declares that he has no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Actis AB and Gallará RV are co-last authors.
Rights and permissions
About this article
Cite this article
Escandriolo Nackauzi, J., Repossi, G., Bernal, C. et al. Dietary fatty acids and the time elapsed from their intake are related to their composition in rat submandibular gland and salivary flow rates. Clin Oral Invest 24, 4123–4131 (2020). https://doi.org/10.1007/s00784-020-03285-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03285-6