Abstract
Purpose
This study investigated the anti-Candida activity and the Shore A hardness of a tissue conditioner (Softone™) modified by incorporation of terpinen-4-ol and cinnamaldehyde.
Material and methods
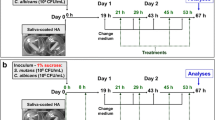
Agar diffusion, microdilution, and mechanism of action methods were performed to determine to evaluate the antifungal activity of phytoconstituents. Then, phytoconstituents in varying concentrations were incorporated into the tissue conditioner. The anti-Candida effect of the modified conditioner was evaluated through agar punch well and biofilm formation methods. Shore A hardness of the experimental liners was evaluated after baseline, 24 h, 48 h, 4 days, and 7 days immersion on artificial saliva.
Results
The phytoconstituents incorporated into Softone showed completely inhibited fungal growth in concentrations of 20–40% and did not present significant antifungal activity until their concentrations where higher than 5%. There were differences between non-modified Softone and M5, M10, C10, and T10% (p < 0.05). The groups containing 10–40% of cinnamaldehyde incorporated into Softone were able to completely inhibit the biofilm. Concentrations below 40% of terpinen-4-ol showed unsatisfactory biofilm inhibition. The T40% and C40% groups presented the lowest Shore A hardness values. Hardness values from groups T40% at 7 days (p = 0.476); C40% at 4 days (p = 0.058); and T20% (p = 0.058), C20% (p = 0.205), T30% (p = 0.154), and C30% (p = 0.874) after 48 h did not differ from the control group.
Conclusions
Cinnamaldehyde incorporated into Softone inhibited Candida biofilm formation at concentrations of 10–40%, being more effective than terpinen-4-ol modification despite of halo inhibition observed by both products.
Clinical relevance
All modifications showed a very similar pattern of hardness being useful for clinical practice.


Similar content being viewed by others
References
Williams D, Lewis M (2011) Pathogenesis and treatment of oral candidosis. J Oral Microbiol 3(1):5771
Wilson J (1998) The aetiology, diagnosis and management of denture stomatitis. Br Dent J 185(8):380–384
Chandra J, Mukherjee PK, Leidich SD, Faddoul FF, Hoyer LL, Douglas LJ, Ghannoum MA (2001) Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J Dent Res 80(3):903–908
Redding S, Bhatt B, Rawls HR, Siegel G, Scott K, Lopez-Ribot J (2009) Inhibition of Candida albicans biofilm formation on denture material. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 107(5):669–672
Pereira CA, Toledo BC, Santos CT, Pereira Costa ACB, Back-Brito GN, Kaminagakura E, Jorge AOC (2013) Opportunistic microorganisms in individuals with lesions of denture stomatitis. Diagn Microbiol Infect Dis 76(4):419–424
Ramage G, Vandewalle K, Wickes BL, López-Ribot JL (2001) Characteristics of biofilm formation by Candida albicans. Rev Iberoam Micol 18(4):163–170
Radnai M, Whiley R, Friel T, Wright PS (2010) Effect of antifungal gels incorporated into a tissue conditioning material on the growth of Candida albicans. Gerodontology 27(4):292–296
Uludamar A, Gökhan Özyeşil A, Ozkan YK (2011) Clinical and microbiological efficacy of three different treatment methods in the management of denture stomatitis. Gerodontology 28(2):104–110
Parker S, Braden M (1990) Formulation of tissue conditioners. Biomaterials. 11(8):579–584
Brown D (1988) Resilient soft liners and tissue conditioners. Br Dent J 164(11):357–360
Malmström HS, Mehta N, Sanchez R, Moss ME (2002) The effect of two different coatings on the surface integrity and softness of a tissue conditioner. J Prosthet Dent 87(2):153–157
Catalán A, Pacheco JG, Martínez A, Mondaca MA (2008) In vitro and in vivo activity of Melaleuca alternifolia mixed with tissue conditioner on Candida albicans. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol 105(3):327–332
Falah-Tafti A, Jafari A, Lotfi-Kamran M, Fallahzadeh H, Hayan R (2010) A comparison of the efficacy of nystatin and fluconazole incorporated into tissue conditioner on the in vitro attachment and colonization of Candida albicans. Dent Res J (Isfahan) 7(1):18–22
Sharma S, Hegde V (2014) Comparative evaluation of antifungal activity of Melaleuca oil and fluconazole when incorporated in tissue conditioner: an in vitro study. J Prosthodont 23(5):367–373
Bueno M, Urban V, Barbério G, da Silva WJ, Porto VC, Pinto LR, Neppelenbroek KH (2015) Effect of antimicrobial agents incorporated into resilient denture relines on the Candida albicans biofilm. Oral Dis 21(1):57–65
Khan N, Shreaz S, Bhatia R, Ahmad SI, Muralidhar S, Manzoor N, Khan LA (2012) Anticandidal activity of curcumin and methyl cinnamaldehyde. Fitoterapia 83(3):434–440
Leite MCA, Bezerra AP de B, Sousa JP, Guerra FQS, Lima EO (2014) Evaluation of antifungal activity and mechanism of action of citral against Candida albicans. Evid Based Complement Alternat Med 1:1–9
Hammer KA, Carson CF, Riley TV (2003) Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. J Appl Microbiol 95(4):853–860
Shreaz S, Bhatia R, Khan N, Muralidhar S, Basir SF, Manzoor N, Khan LA (2011) Spice oil cinnamaldehyde exhibits potent anticandidal activity against fluconazole resistant clinical isolates. Fitoterapia. 82(7):1012–1020
Taguchi Y, Hasumi Y, Hayama K, Arai R, Nishiyama Y, Abe S (2012) Effect of cinnamaldehyde on hyphal growth of C.albicans under various treatment conditions. Med Mycol J 53(3):199–204
Ninomiya K, Maruyama N, Inoue S, Ishibashi H, Takizawa T, Oshima H, Abe S (2012) The essential oil of Melaleuca alternifolia (tea tree oil) and its main component, terpinen-4-ol protect mice from experimental oral candidiasis. Biol Pharm Bull 35(6):861–865
Taguchi Y, Hasumi Y, Abe S, Nishiyama Y (2013) The effect of cinnamaldehyde on the growth and the morphology of Candida albicans. Med Mol Morphol 46(1):8–13
CLSI (2002) Reference method for broth dilution antifungal susceptibility testing of yeasts, 2nd edn. NCCLS, Pennsylvania
CLSI (2009) Reference method for antifungal disk diffusion susceptibility testing of yeasts, 2nd edn, Wayne
Pachava KR, Nadendla L hmi K, Alluri LSC, Tahseen H, Sajja NP (2015) In vitro antifungal evaluation of denture soft liner incorporated with tea tree oil: a new therapeutic approach towards denture stomatitis. J Clin Diagn Res 9(6):ZC62–ZC64
Deswal DP, Chand U (1997) Standardization of the tetrazolium test for viability estimation in ricebean (Vigna umbellata (Thunb.) Ohwi & ohashi) seeds. Seed Sci Technol 25(3):409–417
Aligiannis N, Kalpoutzakis E, Mitaku S, Chinou IB (2001) Composition and antimicrobial activity of the essential oils of two Origanum species. J Agric Food Chem 49(9):4168–4170
Ferreira GLS, Rosalen PL, Peixoto LR, Pérez ALA de L et al (2017) Antibiofilm activity and mechanism of action of the disinfectant chloramine T on Candida spp., and its toxicity against human cells. Molecules 22(9):1527
Escalante A, Gattuso M, Pérez P, Zacchino S (2008) Evidence for the mechanism of action of the antifungal phytolaccoside B isolated from Phytolacca tetramera Hauman. J Nat Prod 71(10):1720–1725
da Silva WJ, Seneviratne J, Parahitiyawa N, Rosa EAR, Samaranayake LP, Cury AADB (2008) Improvement of XTT assay performance for studies involving Candida albicans biofilms. Braz Dent J 19(4):364–369
Bertolini MM, Portela MB, Curvelo JAR, Soares RMA, Lourenço EJV, Telles DM (2014) Resins-based denture soft lining materials modified by chlorhexidine salt incorporation: an in vitro analysis of antifungal activity, drug release and hardness. Dent Mater 30(8):793–798
10139-2 I (2009) Dentristy-soft lining materials for removable dentures
Elsemann R, Cosme D, Souto A (2008) Degradation of tissue conditioners in complete dentures: an in situ study. Int J Prosthodont 21(6):486–488
Siddiqui ZN, Farooq F, Musthafa TNM, Ahmad A, Khan AU (2013) Synthesis, characterization and antimicrobial evaluation of novel halopyrazole derivatives. J Saudi Chem Soc 17(2):237–243
Soliman S, Alnajdy D, El-Keblawy A, Mosa K, Khoder G, Noreddin A (2017) Plants’ natural products as alternative promising anti-Candida drugs. Pharmacogn Rev 11(22):104–122
Zida A, Bamba S, Yacouba A, Ouedraogo-Traore R, Guiguemdé RT (2017) Anti-Candida albicans natural products, sources of new antifungal drugs: a review. J Mycol Med 27(1):1–19
Mothana RAA, Abdo SAA, Hasson S, Althawab FMN, Alaghbari SAZ, Lindequist U (2010) Antimicrobial, antioxidant and cytotoxic activities and phytochemical screening of some Yemeni medicinal plants. Evid Based Complement Alternat Med 7(3):323–330
Abidi SH, Ahmed K, Sherwani SK, Kazmi SU (2015) Synergy between antibiotics and natural agents results in increased antimicrobial activity against Staphylococcus epidermidis. J Infect Dev Ctries 9(9):925–929
Pérez P, Ribas JC (2004) Cell wall analysis. Methods 33(3):245–251
Iqbal Z, Zafar MS (2016) Role of antifungal medicaments added to tissue conditioners: a systematic review. J Prosthodont Res 60(4):231–239
Urban VM, Lima TF, Bueno MG, Giannini M, Arioli Filho JN, de Almeida ALPF, Neppelenbroek KH (2015) Effect of the addition of antimicrobial agents on Shore A hardness and roughness of soft lining materials. J Prosthodont 24(3):207–214
Vankadara S, Hallikerimath RB, Patil V, Bhat K, Doddamani MH (2017) Effect of Melaleuca alternifolia mixed with tissue conditioners in varying doses on colonization and inhibition of Candida albicans: an in vitro study. Contemp Clin Dent 8(3):446–450
Srivatstava A, Ginjupalli K, Perampalli NU, Bhat N, Ballal M (2013) Evaluation of the properties of a tissue conditioner containing origanum oil as an antifungal additive. J Prosthet Dent 110(4):313–319
Geerts GAVM, Stuhlinger ME, Basson NJ (2008) Effect of an antifungal denture liner on the saliva yeast count in patients with denture stomatitis: a pilot study. J Oral Rehabil 35(9):664–669
Lamfon H, Porter SR, McCullough M, Pratten J (2004) Susceptibility of Candida albicans biofilms grown in a constant depth film fermentor to chlorhexidine, fluconazole and miconazole: a longitudinal study. J Antimicrob Chemother 53(2):383–385
Craig R (1997) Prosthetic applications of polymers. Restor Dent Mater 9(1):502–550
Pavan S, Arioli Filho JN, Dos Santos PH, Nogueira SS, Batista AUD (2007) Effect of disinfection treatments on the hardness of soft denture liner materials. J Prosthodont 16(2):101–106
Hong G, Li Y, Maeda T, Mizumachi W, Sadamori S, Hamada T, Murata H (2008) Influence of storage methods on the surface roughness of tissue conditioners. Dent Mater J 27(2):153–158
García-Salinas S, Elizondo-Castillo H, Arruebo M, Mendoza G, Irusta S (2018) Evaluation of the antimicrobial activity and cytotoxicity of different components of natural origin present in essential oils. Molecules 23(6):2–18
Maquera-Huacho PM, Tonon CC, Correia MF, Francisconi RS, Bordini EAF, Marcantonio É, Spolidorio DMP (2018) In vitro antibacterial and cytotoxic activities of carvacrol and terpinen-4-ol. Biofouling 34(6):1–11
Funding
All the investment to carry out the research was being of all the responsibilities of the researchers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Fátima Souto Maior, L., Maciel, P.P., Ferreira, V.Y.N. et al. Antifungal activity and Shore A hardness of a tissue conditioner incorporated with terpinen-4-ol and cinnamaldehyde. Clin Oral Invest 23, 2837–2848 (2019). https://doi.org/10.1007/s00784-019-02925-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-02925-w