Abstract.
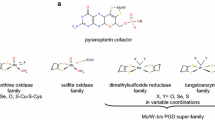
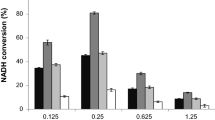
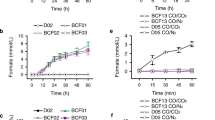
Carbon monoxide dehydrogenase (CODH) from Rhodospirillum rubrum reversibly catalyzes the oxidation of CO to CO2 at the active site C-cluster. In this article, the reduction of CO2 to formate is reported as a slow side reaction catalyzed by both Ni-containing CODH and Ni-deficient CODH. Recently, the structures of R. rubrum CODH and its active site NiFeS cluster (the C-cluster) have been solved. The data in this manuscript describe the formate-producing capability of CODH with or without Ni in the active site.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Heo, J., Skjeldal, L., Staples, C.R. et al. Carbon monoxide dehydrogenase from Rhodospirillum rubrum produces formate. J Biol Inorg Chem 7, 810–814 (2002). https://doi.org/10.1007/s00775-002-0365-z
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00775-002-0365-z