Abstract
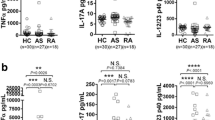
Ankylosing spondylitis (AS) is characterized by excessive bone formation with syndesmophytes, leading to bony ankylosis. The contribution of osteoblasts to the pathogenesis of ankylosis is poorly understood. The aim of this study was to determine molecular differences between disease controls (Ct) and AS bone-derived cells (BdCs) during osteogenic differentiation with or without inflammation using AS patient serum. We confirmed osteoblastic differentiation of Ct and AS BdCs under osteogenic medium by observing morphological changes and measuring osteoblastic differentiation markers. Osteoblast differentiation was detected by alkaline phosphatase (ALP) staining and activity, and alizarin red and hydroxyapatite staining. Osteoblast-specific markers were analyzed by quantitative reverse-transcriptase-polymerase chain reaction, immunoblotting, and immunostaining. To examine the effects of inflammation, we added AS and healthy control serum to Ct and AS BdCs, and then analyzed osteoblast-specific markers. AS BdCs showed elevated basal intercellular and extracellular ALP activity compared to Ct. When osteoblast differentiation was induced, AS BdCs exhibited higher expression of osteoblast-specific marker genes and faster mineralization than Ct, indicating that these cells differentiated more rapidly into osteoblasts. ALP activity and mineralization accelerated when serum from AS patients was added to Ct and AS BdCs. Our results revealed that AS BdCs showed significantly increased osteoblastic activity and differentiation capacity by regulating osteoblast-specific transcription factors and proteins compared to Ct BdCs. Active inflammation of AS serum accelerated osteoblastic activity. Our study could provide useful basic data for understanding the molecular mechanism of ankylosis in AS.


Similar content being viewed by others
References
Xie Z, Wang P, Li Y, Deng W, Zhang X, Su H, Li D, Wu Y, Shen H (2016) Imbalance between bone morphogenetic protein 2 and noggin induces abnormal osteogenic differentiation of mesenchymal stem cells in ankylosing spondylitis. Arthritis Rheumatol 68:430–440
Xie Z, Li J, Wang P, Li Y, Wu X, Wang S, Su H, Deng W, Liu Z, Cen S, Ouyang Y, Wu Y, Shen H (2016) Differential expression profiles of long noncoding RNA and mRNA of osteogenically differentiated mesenchymal stem cells in ankylosing spondylitis. J Rheumatol 43:1523–1531
Komori T (2006) Regulation of osteoblast differentiation by transcription factors. J Cell Biochem 99:1233–1239
Beck GR Jr, Zerler B, Moran E (2000) Phosphate is a specific signal for induction of osteopontin gene expression. Proc Natl Acad Sci USA 97:8352–8357
Witkowska-Zimny M, Walenko K, Wrobel E, Mrowka P, Mikulska A, Przybylski J (2013) Effect of substrate stiffness on the osteogenic differentiation of bone marrow stem cells and bone-derived cells. Cell Biol Int 37:608–616
Helfrich MH, Ralston S (2003) Bone research protocols. Methods in molecular medicine, vol 80. Humana Press, Totowa, pp 3–18 (xiv, 448)
Taylor SE, Shah M, Orriss IR (2014) Generation of rodent and human osteoblasts. Bonekey Rep 3:585
Kim HR, Lee SH, Kim HY (2006) Elevated serum levels of soluble receptor activator of nuclear factors-kappaB ligand (sRANKL) and reduced bone mineral density in patients with ankylosing spondylitis (AS). Rheumatology (Oxford) 45:1197–1200
Ducy P, Starbuck M, Priemel M, Shen J, Pinero G, Geoffroy V, Amling M, Karsenty G (1999) A Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes Dev 13:1025–1036
Jo S, Lee YL, Kim S, Lee H, Chung H (2016) PCGF2 negatively regulates arsenic trioxide-induced PML-RARA protein degradation via UBE2I inhibition in NB4 cells. Biochim Biophys Acta 1863:1499–1509
Acknowledgements
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future (NRF-2016R1A2B4008606).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Jo, S., Kang, S., Han, J. et al. Accelerated osteogenic differentiation of human bone-derived cells in ankylosing spondylitis. J Bone Miner Metab 36, 307–313 (2018). https://doi.org/10.1007/s00774-017-0846-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-017-0846-3