Abstract
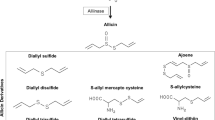
Natural organosulfur compounds (OSCs) have been shown to have chemopreventive effects and to suppress the proliferation of tumor cells in vitro through the induction of apoptosis. The biochemical mechanisms underlying the antitumorigenic and anti-proliferative effects of garlic-derived OSCs are not fully understood. Several modes of action of these compounds have been proposed, and it seems likely that the rate of clearance of allyl sulfur groups from cells is a determinant of the overall response. The aim of this review is to focus attention on the effects of natural allyl sulfur compounds on the cell detoxification system in normal and tumor cells. It has been already reported that several natural allyl sulfur compounds induce chemopreventive effects by affecting xenobiotic metabolizing enzymes and inducing their down-activation. Moreover, different effects of water- and oil-soluble allyl sulfur compounds on enzymes involved in the detoxification system of rat tissues have been observed. A direct interaction of the garlic allyl sulfur compounds with proteins involved in the detoxification system was studied in order to support the hypothesis that proteins possessing reactive thiol groups and that are involved in the detoxification system and in the cellular redox homeostasis, are likely the preferential targets of these compounds. The biochemical transformation of the OSCs in the cell and their adducts with thiol functional groups of these proteins, could be considered relevant events to uncover the anticancer properties of the allyl sulfur compounds. Although additional studies, using proteomic approaches and transgenic models, are needed to identify the molecular targets and modes of action of these natural compounds, the allyl sulfur compounds can represent potential ideal agents in anticancer therapy, either alone or in association with other antitumor drugs.





Similar content being viewed by others
Abbreviations
- 2-PTS:
-
Sodium-2-propenyl-thiosulfate
- AMS:
-
Allyl methyl sulfide
- BBM:
-
Brush-border membranes
- CAR:
-
Constitutive androstane receptor
- GS-DNB:
-
Glutathione-2,4-dinitrobenzene conjugate
- DAS:
-
Diallyl sulfide
- DADS:
-
Diallyl disulfide
- DASO:
-
Diallyl sulfoxide
- DASO2 :
-
Diallyl sulfone
- DATS:
-
Diallyl trisulfide
- DCF-DA:
-
2′,7′-Dichlorodihydrofluorescein diacetate
- DMDS:
-
Dimethyl disulfide
- DMH:
-
Dimethyl-hydrazine
- DTT:
-
Dithiothreitol
- GSH:
-
Glutathione
- GST:
-
Glutathione S-transferase
- HO 1:
-
Heme oxygenase 1
- MDR:
-
Multidrug resistance
- MRP:
-
Multidrug resistance protein
- MST:
-
3-Mercaptopyruvate sulfurtransferase
- NQO1:
-
NAD(P)H: quinone oxidoreductase 1
- Nrf2:
-
NF-E2-related factor-2
- Oatp4:
-
Organic anion transporting polypeptide 4
- OSCs:
-
Organosulfur compounds
- P450 s:
-
Cytochrome P450 enzymes
- P-gp:
-
P-glycoprotein
- ROS:
-
Reactive oxygen species
- SAC:
-
S-allylcysteine
- SAMC:
-
S-allylmercaptocysteine
- Trd:
-
Thioredoxin reductase
- Trx:
-
Thioredoxin
- TST:
-
Thiosulfate sulfurtransferase
- UGT:
-
UDP-glucuronosyl transferase
References
Aminlari M, Gilanpour H (1991) Comparative studies on the distribution of rhodanese in different tissues of domestic animals. Comp Biochem Physiol B 99:673–677
Aminlari M, Gholami S, Vaseghi T, Azarafrooz A (1997) Rhodanese (thiosulfate: cyanide sulfurtransferase) in the digestive tract of chicken at different stages of development. Poult Sci 76:318–320
Andorfer JH, Tchaikovskaya T, Listowsky I (2004) Selective expression of glutathione S-transferase genes in the murine gastrointestinal tract in response to dietary organosulfur compounds. Carcinogenesis 25:359–367
Arnold KA, Eichelbaum M, Burk O (2004) Alternative splicing affects the function and tissue-specific expression of the human constitutive androstane receptor. Nucl Recept 2:1
Arora A, Seth K, Shukla Y (2004) Reversal of P-glycoprotein-mediated multidrug resistance by diallyl sulfide in K562 leukemic cells and in mouse liver. Carcinogenesis 25:941–949
Battistoni A, Mazzetti AP, Petruzzelli R, Muramatsu M, Federici G, Ricci G, Lo Bello M (1995) Cytoplasmic and periplasmic production of human placental glutathione transferase in Escherichia coli. Protein Expr Purif 6:579–587
Borst P, Evers R, Kool M, Wijnholds J (2000) A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst 92:1295–1302
Bose C, Guo J, Zimniak L, Srivastava SK, Singh SP, Zimniak P, Singh SV (2002) Critical role of allyl groups and disulfide chain in induction of Pi class glutathione transferase in mouse tissues in vivo by diallyl disulfide, a naturally occurring chemopreventive agent in garlic. Carcinogenesis 23:1661–1665
Boyer TD, Kenney WC (1985) Acidic glutathione S-transferases of rat testis. Biochem J 230:125–132
Brady JF, Ishizaki H, Fukuto JM, Lin MC, Fadel A, Gapac JM, Yang CS (1991a) Inhibition of cytochrome P-450 2E1 by diallyl sulfide and its metabolites. Chem Res Toxicol 4:642–647 (a)
Brady JF, Wang MH, Hong JY, Xiao F, Li Y, Yoo JS, Ning SM, Lee MJ, Fukuto JM, Gapac JM et al (1991b) Modulation of rat hepatic microsomal monooxygenase enzymes and cytotoxicity by diallyl sulfide. Toxicol Appl Pharmacol 108:342–354
Buck ML (1997) The cytochrome P450 enzyme system and its effect on drug metabolism. Pediatr Pharmacother 3(5):211–216
Chen C, Pung D, Leong V, Hebbar V, Shen G, Nair S, Li W, Kong AN (2004) Induction of detoxifying enzymes by garlic organosulfur compounds through transcription factor Nrf2: effect of chemical structure and stress signals. Free Radic Biol Med 37:1578–1590
Cooper AJ, Pinto JT (2005) Aminotransferase, l-amino acid oxidase and beta-lyase reactions involving l-cysteine S-conjugates found in allium extracts. Relevance to biological activity? Biochem Pharmacol 69:209–220
Davenport DM, Wargovich MJ (2005) Modulation of cytochrome P450 enzymes by organosulfur compounds from garlic. Food Chem Toxicol 43:1753–1762
Demeule M, Brossard M, Turcotte S, Regina A, Jodoin J, Beliveau R (2004) Diallyl disulfide, a chemopreventive agent in garlic, induces multidrug resistance-associated protein 2 expression. Biochem Biophys Res Commun 324:937–945
Dirsch VM, Gerbes AL, Vollmar AM (1998) Ajoene, a compound of garlic, induces apoptosis in human promyeloleukemic cells, accompanied by generation of reactive oxygen species and activation of nuclear factor kappaB. Mol Pharmacol 53:402–407
Dragnev KH, Nims RW, Lubet RA (1995) The chemopreventive agent diallyl sulfide. A structurally atypical phenobarbital-type inducer. Biochem Pharmacol 50:2099–2104
Dwivedi C, Abu-Ghazaleh A, Guenther J (1996) Effects of diallyl sulfide and diallyl disulfide on cisplatin-induced changes in glutathione and glutathione-S-transferase activity. Anticancer Drugs 7:792–794
Evers R, Kool M, van Deemter L, Janssen H, Calafat J, Oomen LC, Paulusma CC, Oude Elferink RP, Baas F, Schinkel AH, Borst P (1998) Drug export activity of the human canalicular multispecific organic anion transporter in polarized kidney MDCK cells expressing cMOAT (MRP2) cDNA. J Clin Invest 101:1310–1319
Fisher CD, Augustine LM, Maher JM, Nelson DM, Slitt AL, Klaassen CD, Lehman-McKeeman LD, Cherrington NJ (2007) Induction of drug-metabolizing enzymes by garlic and allyl sulfide compounds via activation of constitutive androstane receptor and nuclear factor E2-related factor 2. Drug Metab Dispos 35:995–1000
Fukao T, Hosono T, Misawa S, Seki T, Ariga T (2004) The effects of allyl sulfides on the induction of phase II detoxification enzymes and liver injury by carbon tetrachloride. Food Chem Toxicol 42:743–749
Gottesman MM, Pastan I (1993) Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem 62:385–427
Gurtoo HL, Marinello AJ, Struck RF, Paul B, Dahms RP (1981) Studies on the mechanism of denaturation of cytochrome P-450 by cyclophosphamide and its metabolites. J Biol Chem 256:11691–11701
Guyonnet D, Siess MH, Le Bon AM, Suschetet M (1999) Modulation of phase II enzymes by organosulfur compounds from Allium vegetables in rat tissues. Toxicol Appl Pharm 154:50–58
Guyonnet D, Belloir C, Suschetet M, Siess MH, Le Bon AM (2000) Liver subcellular fractions from rats treated by organosulfur compounds from Allium modulate mutagen activation. Mutat Res 466:17–26
Haber D, Siess MH, Canivenc-Lavier MC, Le Bon AM, Suschetet M (1995a) Differential effects of dietary diallyl sulfide and diallyl disulfide on rat intestinal and hepatic drug-metabolizing enzymes. J Toxicol Environ Health 44:423–434
Haber D, Siess MH, Canivenc-Lavier MC, Le Bon AM, Suschetet M (1995b) Differential effects of dietary diallyl sulfide and diallyl disulfide on rat intestinal and hepatic drug-metabolizing enzymes. J Toxicol Environ Health 44:423–434
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hatono S, Jimenez A, Wargovich MJ (1996) Chemopreventive effect of S-allylcysteine and its relationship to the detoxification enzyme glutathione S-transferase. Carcinogenesis 17:1041–1044
Hayes JD, Pulford DJ (1995) The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol 30:445–600
Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA (2003) Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425:191–196
Hu X, Singh SV (1997) Glutathione S-transferases of female A/J mouse lung and their induction by anticarcinogenic organosulfides from garlic. Arch Biochem Biophys 340:279–286
Hu X, Benson PJ, Srivastava SK, Mack LM, Xia H, Gupta V, Zaren HA, Singh SV (1996) Glutathione S-transferases of female A/J mouse liver and forestomach and their differential induction by anti-carcinogenic organosulfides from garlic. Arch Biochem Biophys 336:199–214
Huang W, Zhang J, Chua SS, Qatanani M, Han Y, Granata R, Moore DD (2003) Induction of bilirubin clearance by the constitutive androstane receptor (CAR). Proc Natl Acad Sci USA 100:4156–4161
Iciek M, Bilska A, Ksiazek L, Srebro Z, Wlodek L (2005) Allyl disulfide as donor and cyanide as acceptor of sulfane sulfur in the mouse tissues. Pharmacol Rep 57:212–218
Ishikawa T, Ali-Osman F (1993) Glutathione-associated cis-diamminedichloroplatinum(II) metabolism and ATP-dependent efflux from leukemia cells. Molecular characterization of glutathione–platinum complex and its biological significance. J Biol Chem 268:20116–20125
Jin L, Baillie TA (1997) Metabolism of the chemoprotective agent diallyl sulfide to glutathione conjugates in rats. Chem Res Toxicol 10:318–327
Knowles LM, Milner JA (1998) Depressed p34cdc2 kinase activity and G2/M phase arrest induced by diallyl disulfide in HCT-15 cells. Nutr Cancer 30:169–174
Le Bon AM, Vernevaut MF, Guenot L, Kahane R, Auger J, Arnault I, Haffner T, Siess MH (2003) Effects of garlic powders with varying alliin contents on hepatic drug metabolizing enzymes in rats. J Agric Food Chem 51:7617–7623
Lea MA (1996) Organosulfur compounds and cancer. Adv Exp Med Biol 401:147–154
Lea MA, Randolph VM, Patel M (1999) Increased acetylation of histones induced by diallyl disulfide and structurally related molecules. Int J Oncol 15:347–352
Li G, Qiao CH, Lin RI, Pinto J, Osborne MP, Tiwari RK (1995) Anti-proliferative effects of garlic constituents in cultured human breast cancer cells. Oncol Rep 2:787–791
Lo Bello M, Parker MW, Desideri A, Polticelli F, Falconi M, Del Boccio G, Pennelli A, Federici G, Ricci G (1993) Peculiar spectroscopic and kinetic properties of Cys-47 in human placental glutathione transferase. Evidence for an atypical thiolate ion pair near the active site. J Biol Chem 268:19033–19038
Lo Bello M, Battistoni A, Mazzetti AP, Board PG, Muramatsu M, Federici G, Ricci G (1995) Site-directed mutagenesis of human glutathione transferase P1–1. Spectral, kinetic, and structural properties of Cys-47 and Lys-54 mutants. J Biol Chem 270:1249–1253
Malayeri R, Filipits M, Suchomel RW, Zochbauer S, Lechner K, Pirker R (1996) Multidrug resistance in leukemias and its reversal. Leuk Lymphoma 23:451–458
Mannervik B, Danielson UH (1988) Glutathione transferases—structure and catalytic activity. CRC Crit Rev Biochem 23:283–337
Mannervik B, Alin P, Guthenberg C, Jensson H, Tahir MK, Warholm M, Jornvall H (1985) Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci USA 82:7202–7206
Meyer JM, Rodvold KA (1996) Drug biotransformation by the cytochrome P-450 enzyme system. Infect Med 13(6):452, 459, 463–464, 523
Munday R, Munday CM (1999) Low doses of diallyl disulfide, a compound derived from garlic, increase tissue activities of quinone reductase and glutathione transferase in the gastrointestinal tract of the rat. Nutr Cancer 34:42–48
Nian H, Delage B, Pinto JT, Dashwood RH (2008) Allyl mercaptan, a garlic-derived organosulfur compound, inhibits histone deacetylase and enhances Sp3 binding on the P21WAF1 promoter. Carcinogenesis 29:1816–1824
Pagani S, Bonomi F, Cerletti P (1984) Enzymic synthesis of the iron-sulfur cluster of spinach ferredoxin. Eur J Biochem 142:361–366
Pan J, Hong JY, Ma BL, Ning SM, Paranawithana SR, Yang CS (1993) Transcriptional activation of cytochrome P450 2B1/2 genes in rat liver by diallyl sulfide, a compound derived from garlic. Arch Biochem Biophys 302:337–342
Paulusma CC, Oude Elferink RP (1997) The canalicular multispecific organic anion transporter and conjugated hyperbilirubinemia in rat and man. J Mol Med 75:420–428
Phillips MF, Mantle TJ (1993) Inactivation of mouse liver glutathione S-transferase YfYf (Pi class) by ethacrynic acid and 5, 5′-dithiobis-(2-nitrobenzoic acid). Biochem J 294(Pt 1):57–62
Pinto JT, Qiao C, Xing J, Rivlin RS, Protomastro ML, Weissler ML, Tao Y, Thaler H, Heston WD (1997) Effects of garlic thioallyl derivatives on growth, glutathione concentration, and polyamine formation of human prostate carcinoma cells in culture. Am J Clin Nutr 66:398–405
Reicks MM, Crankshaw DL (1996) Modulation of rat hepatic cytochrome P-450 activity by garlic organosulfur compounds. Nutr Cancer 25:241–248
Rosen RT, Hiserodt RD, Fukuda EK, Ruiz RJ, Zhou Z, Lech J, Rosen SL, Hartman TG (2001) Determination of allicin, S-allylcysteine and volatile metabolites of garlic in breath, plasma or simulated gastric fluids. J Nutr 131:968S–971S
Sabelli R, Iorio E, De Martino A, Podo F, Ricci A, Viticchie G, Rotilio G, Paci M, Melino S (2008) Rhodanese-thioredoxin system and allyl sulfur compounds. Febs J 275:3884–3899
Sakamoto K, Lawson LD, Milner JA (1997) Allyl sulfides from garlic suppress the in vitro proliferation of human A549 lung tumor cells. Nutr Cancer 29:152–156
Scharfenberg K, Wagner R, Wagner KG (1990) The cytotoxic effect of ajoene, a natural product from garlic, investigated with different cell lines. Cancer Lett 53:103–108
Schaub TP, Kartenbeck J, Konig J, Vogel O, Witzgall R, Kriz W, Keppler D (1997) Expression of the conjugate export pump encoded by the mrp2 gene in the apical membrane of kidney proximal tubules. J Am Soc Nephrol 8:1213–1221
Sheen LY, Sheu SF, Tsai SJ, Meng RH, Lii CK (1999) Effect of garlic active principle, diallyl disulfide, on cell viability, lipid peroxidation, glutathione concentration and its related enzyme activities in primary rat hepatocytes. Am J Chin Med 27:95–105
Shenoy NR, Choughuley AS (1992) Inhibitory effect of diet related sulphydryl compounds on the formation of carcinogenic nitrosamines. Cancer Lett 65:227–232
Shirin H, Pinto JT, Kawabata Y, Soh JW, Delohery T, Moss SF, Murty V, Rivlin RS, Holt PR, Weinstein IB (2001) Antiproliferative effects of S-allylmercaptocysteine on colon cancer cells when tested alone or in combination with sulindac sulfide. Cancer Res 61:725–731
Sigounas G, Hooker JL, Li W, Anagnostou A, Steiner M (1997) S-allylmercaptocysteine, a stable thioallyl compound, induces apoptosis in erythroleukemia cell lines. Nutr Cancer 28:153–159
Singh SV, Pan SS, Srivastava SK, Xia H, Hu X, Zaren HA, Orchard JL (1998) Differential induction of NAD(P)H:quinone oxidoreductase by anti-carcinogenic organosulfides from garlic. Biochem Biophys Res Commun 244:917–920
Sörbo BH (1953) Crystalline bovine rhodanese and its properties. Acta Chem Scand 7:1129–1136
Sparnins VL, Mott AW, Barany G, Wattenberg LW (1986) Effects of allyl methyl trisulfide on glutathione S-transferase activity and BP-induced neoplasia in the mouse. Nutr Cancer 8:211–215
Sparnins VL, Barany G, Wattenberg LW (1988) Effects of organosulfur compounds from garlic and onions on benzo[a]pyrene-induced neoplasia and glutathione S-transferase activity in the mouse. Carcinogenesis 9:131–134
Sumiyoshi H, Wargovich MJ (1990) Chemoprevention of 1, 2-dimethylhydrazine-induced colon cancer in mice by naturally occurring organosulfur compounds. Cancer Res 50:5084–5087
Sundaram SG, Milner JA (1993) Impact of organosulfur compounds in garlic on canine mammary tumor cells in culture. Cancer Lett 74:85–90
Sundaram SG, Milner JA (1996) Diallyl disulfide induces apoptosis of human colon tumor cells. Carcinogenesis 17:669–673
Takeyama H, Hoon DS, Saxton RE, Morton DL, Irie RF (1993) Growth inhibition and modulation of cell markers of melanoma by S-allyl cysteine. Oncology 50:63–69
Tew KD (1994) Glutathione-associated enzymes in anticancer drug resistance. Cancer Res 54:4313–4320
Toohey JI (1989) Sulphane sulphur in biological systems: a possible regulatory role. Biochem J 264:625–632
Tsai CW, Chen HW, Yang JJ, Sheen LY, Lii CK (2007) Diallyl disulfide and diallyl trisulfide up-regulate the expression of the pi class of glutathione S-transferase via an AP-1-dependent pathway. J Agric Food Chem 55:1019–1026
Tsuchida S, Sato K (1992) Glutathione transferases and cancer. Crit Rev Biochem Mol Biol 27:337–384
Welch C, Wuarin L, Sidell N (1992) Antiproliferative effect of the garlic compound S-allyl cysteine on human neuroblastoma cells in vitro. Cancer Lett 63:211–219
Westley J (1973) Rhodanese. Adv Enzymol Relat Areas Mol Biol 39:327–368
Westley AM, Westley J (1989) Voltammetric determination of cyanide and thiocyanate in small biological samples. Anal Biochem 181:190–194
Wilce MC, Parker MW (1994) Structure and function of glutathione S-transferases. Biochim Biophys Acta 1205:1–18
Wu CC, Sheen LY, Chen HW, Tsai SJ, Lii CK (2001) Effects of organosulfur compounds from garlic oil on the antioxidation system in rat liver and red blood cells. Food Chem Toxicol 39:563–569
Wu CC, Sheen LY, Chen HW, Kuo WW, Tsai SJ, Lii CK (2002) Differential effects of garlic oil and its three major organosulfur components on the hepatic detoxification system in rats. J Agric Food Chem 50:378–383
Xiao D, Pinto JT, Soh JW, Deguchi A, Gundersen GG, Palazzo AF, Yoon JT, Shirin H, Weinstein IB (2003) Induction of apoptosis by the garlic-derived compound S-allylmercaptocysteine (SAMC) is associated with microtubule depolymerization and c-Jun NH(2)-terminal kinase 1 activation. Cancer Res 63:6825–6837
Yang CS, Wang ZY, Hong JY (1994) Inhibition of tumorigenesis by chemicals from garlic and tea. Adv Exp Med Biol 354:113–122
Acknowledgments
We thank Prof. Anna Maria Caccuri for giving us the recombinant GST1π-1 protein, Dr.Giuditta Viticchiè for technical support in some experiments and Dr. Gaetano Barbato for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Melino, S., Sabelli, R. & Paci, M. Allyl sulfur compounds and cellular detoxification system: effects and perspectives in cancer therapy. Amino Acids 41, 103–112 (2011). https://doi.org/10.1007/s00726-010-0522-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-010-0522-6