Abstract
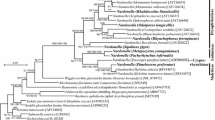
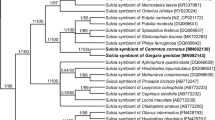
In this study, we surveyed the bacteriome-associated microbiota of the corn leafhopper Dalbulus maidis by means of histological, ultrastructural, and molecular analyses. Amplification and sequencing of 16S rDNA genes revealed that the endosymbiont “Candidatus Sulcia muelleri” (Phylum Bacteroidetes) resides in bacteriomes of D. maidis. Phylogenetic analysis showed that the sequence was closely allied to others found in representatives of the subfamily Deltocephalinae. We failed to amplify other sequences as “Candidatus Nasuia deltocephalinicola,” a co-primary symbiont frequently associated to deltocephaline leafhoppers. In addition, a metagenetic analysis carried out in order to investigate the presence of other bacteriome-associated bacteria of D. maidis showed that the sequence of Sulcia accounted for 98.56 % of all the sequences. Histological and ultrastructural observations showed that microorganisms harbored in bacteriomes (central syncytium and cytoplasm of uninucleate bacteriocytes) look like others Sulcia described in hemipteran species and they were transovarially transmitted from mother to offspring which is typical of obligate endosymbionts. The only presence of Sulcia in the bacteriomes of D. maidis was discussed.






Similar content being viewed by others
References
Baumann P (2005) Biology bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu Rev Microbiol 59:155–189
Baumann P, Moran NA, Baumann L (2006) Bacteriocyte-associated endosymbionts of insects. Prokaryotes 1:403–438
Bennett GM, Moran NA (2013) Small, smaller, smallest: the origins and evolution of ancient dual symbioses in a Phloem-feeding insect. Genome Biol Evol 5:1675–1688
Bennett GM, Abbà S, Kube M, Marzachì C (2016) Complete genome sequences of the obligate symbionts “Candidatus Sulcia muelleri” and “Ca. Nasuia deltocephalinicola” from the pestiferous leafhopper Macrosteles quadripunctulatus (Hemiptera: Cicadellidae). Genome Announc 4:e01604–e01615. doi:10.1128/genomeA.01604-15
Buchner P (1965) Endosymbiosis of animals with plant microorganisms. Interscience Publishers, New York
Carloni E, Carpane P, Paradell S, Laguna I, Giménez Pecci MP (2013) Presence of Dalbulus maidis (Hemiptera: Cicadellidae) and of Spiroplasma kunkelii in the temperate region of Argentina. J Econ Entomol 106:1574–1581
Chang KP, Musgrave AJ (1972) Multiple symbiosis in a leafhopper, Helochara communis Fitch (Cicadellidae: Homoptera): envelopes, nucleoids and inclusions of the symbiotes. J Cell Sci 11:275–293
Chang H, Cho ST, Canale MC, Mugford ST, Lopes JR, Hogenhout SA, Kuo CH (2015) Complete genome sequence of “Candidatus Sulcia muelleri” ML, an obligate nutritional symbiont of maize leafhopper (Dalbulus maidis). Genome Announc 3:e01483–14. doi:10.1128/genomeA.01483–14
Darriba D, Taboada GL, Doallo R, Posada D (2012) Model Test 2: more models, new heuristics and parallel computing. Nat Methods 9:772
DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL (2006) Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72:5069–5072
Felsenstein J (1985) Confidence limits on phylogenies: an approach using bootstrap. Evolution 39:783–791
Galindo Miranda N (1994) Los micetomas, un análisis morfofisiológico de su interacción con los Cicadellidae (Homoptera). Folia Entomol Mex 92:1–8
Giménez Pecci MP, Laguna I, Ávila AO, de Remes Lenicov AMM, Virla E, Borgogno CF, Nome G, Paradell S (2002) Difusión del Corn Stunt Spiroplasma del maíz (Spiroplasma kunkelii) y del vector (Dalbulus maidis) en la República Argentina. Rev Fac Agron La Plata 105:1–8
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Ishii Y, Matsuura Y, Kakizawa S, Nikoh N, Fukatsua T (2013) Diversity of bacterial endosymbionts associated with Macrosteles leafhoppers vectoring phytopathogenic phytoplasmas. App Environ Microbiol 79:5013–5022
Kaiser B (1980) Licht- und elektronenmikroskopische unter-suchlung der symbioten von Graphocephala coccinea Forstier (Homoptera: Jassidae). J Insect Morphol Embryol 9:79–88
Kobialka M, Michalik A, Walczak M, Lz J, Szklarzewicz T (2015a) Sulcia symbiont of the leafhopper Macrosteles laevis (Ribaut, 1927) (Insecta: Hemiptera:Cicadellidae: Deltocephalinae) harbors Arsenophonus bacteria. Protoplasma 253:903–912
Kobialka M, Michalik A, Walczak M, Lz J, Szklarzewicz T (2015b) Symbiotic microorganisms of the leafhopper Deltocephalus pulicaris (Fallén, 1806) (Insecta: Hemiptera: Cicadellidae: Deltocephalinae): molecular characterization, ultrastructure and transovarial transmission. Pol J Entomol 84:289–304
Koga R, Bennett G, Cryan JR, Moran NA (2013) Evolutionary replacement of obligate symbionts in an ancient and diverse insect lineage. Environ Microbiol 15:2073–2081
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA et al (2007) ClustalW and ClustalX version 2. Bioinformatics 23:2947–2948
Marzorati M, Alma A, Sacchi L, Pajoro M et al (2006) A novel Bacteroidetes symbiont is localized in Scaphoideus titanus, the insect vector of Flavescence dorée in Vitis vinifera. Appl Environ Microbiol 72:1467–1475
McCutcheon JP, Moran NA (2007) Parallel genomic evolution and metabolic interdependence in an ancient symbiosis. Proc Natl Acad Sci U S A 104:19392–19397
McCutcheon JP, Moran NA (2010) Functional convergence in reduced genomes of bacterial symbionts spanning 200 My of evolution. Genome Biol Evol 2:708–718
McCutcheon JP, McDonald BR, Moran NA (2009) Convergent evolution of metabolic roles in bacterial co-symbionts of insects. Proc Natl Acad Sci U S A 106:15394–15399
Michalik A, Jankowska W, Szklarzewicz T (2009) Ultrastructure and transovarial transmission of endosymbiotic microorganisms in Conomelus anceps and Metcalfa pruinosa (Insecta: Hemiptera: Fulgoromorpha). Folia Biol (Kraków) 57:131–137
Michalik A, Jankowska W, Kot M, Gołas A, Szklarzewicz T (2014) Symbiosis in the green leafhopper, Cicadella viridis (Hemiptera, Cicadellidae). Association in statu nascendi? Arthropod Struct Dev 43:579–587
Moran NA (1998) Bacteriocyte-associated symbionts of insects. Bioscience 48:295–304
Moran NA (2007) Symbiosis as an adaptive process and source of phenotypic complexity. Proc Natl Acad Sci U S A 104:8627–8633
Moran NA, Dale C, Dunbar H, Smith WA, Ochman H (2003) Intracellular symbionts of sharpshooters (Insecta: Hemiptera: Cicadellinae) form a distinct clade with a small genome. Environ Microbiol 5:116–126
Moran NA, Tran P, Gerardo NM (2005) Symbiosis and insect diversification: an ancient symbiont of sap-feeding insects from the bacterial Phylum Bacteroidetes. Appl Environ Microbiol 71:8802–8810
Nault LR (1990) Evolution of an insect pest: maize and the corn leafhopper, a case study. Maydica 35:165–175
Nault LR, Ammar D (1989) Leafhopper and planthopper transmission of plant virus. Annu Rev Entomol 34:503–529
Noda H (1977) Histological and histochemical observation of intracellular yeast-like symbiotes in the fat body of the small brown planthopper, Laodelphax striatellus (Homoptera: Delphacidae). Appl Entomol Zool 12:134–141
Noda H, Watanabe K, Kawai S, Yukuhiro F, Miyoshi T, Tomizawa M (2012) Bacteriome-associated endosymbionts of the green rice leafhopper Nephotettix cincticeps (Hemiptera: Cicadellidae). Appl Entomol Zool 47:217–225
Oliveira E, Waquil JM, Fernandes FT, Paiva E, Resende RO, Kitajima EW (1998) Enfezamento pàlido e enfezamento vermelho na cultura do milho no Brasil Central. Fitopatol Bras 23:45–47
Sacchi L, Genchi M, Clementi E, Bigliardi E, Avanzati AM, Pajoro M, Negri I, Marzorati M, Gonella E, Alma A (2008) Multiple symbiosis in the leafhopper Scaphoideus titanus (Hemiptera: Cicadellidae): details of transovarial transmission of Cardinium sp. and yeast-like endosymbionts. Tissue Cell 40:231–242
Sandstrom J, Moran NA (1999) How nutritionally imbalanced is phloem sap for aphids? Entomol Exp Appl 91:203–210
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci U S A 74:5463–5467
Summers CG, Newton AS, Opgenorth DC (2004) Overwintering of corn leafhopper, Dalbulus maidis (Homoptera: Cicadellidae), and Spiroplasma kunkelii (Mycoplasmatales: Spiroplasmataceae) in California’s San Joaquin Valley. Environ Entomol 33:1644–1651
Szklarzewicz T, Grzywacz B, Szwedo J, Michalik A (2016) Bacterial symbionts of the leafhopper Evacanthus interruptus (Linnaeus, 1758) (Insecta: Hemiptera: Cicadellidae: Evacanthinae). Protoplasma 253:379–391
Takiya D, Tran P, Dietrich C, Moran N (2006) Co-cladogenesis spanning three phyla: leafhoppers (Insecta: Hemiptera: Cicadellidae) and their dual bacterial symbionts. Mol Ecol 15:4175–4191
Talavera G, Castresana J (2007) Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol 56:564–577
Tsai JH, Perrier JL (1996) Morphology of the digestive and reproductive systems of Dalbulus maidis and Graminella nigrifrons (Homoptera: Cicadellidae). Fla Entomol 79:563–578
Urban JM, Cryan JR (2012) Two ancient bacterial endosymbionts have coevolved with the planthoppers (Insect: Hemiptera: Fulgoroidea). BMC Evol Biol 12:87
Virla E, Díaz C, Carpane P, Laguna I, Ramallo J, Gómez L, Giménez Pecci MP (2004) Evaluación preliminar de la disminución en la producción de maíz causada por el “Corn Stunt Spiroplasma” (CSS) en Tucumán, Argentina. Bol San Veg Plagas 30:403–413
Wangkeeree J, Miller T, Hanboonsong Y (2012) Candidates for symbiotic control of sugarcane white leaf disease. Appl Environ Microbiol 78:6804–6811
Wu D, Daugherty SC, Van Aken SE, Pai GH et al (2006) Metabolic complementarity and genomics of the dual bacterial symbiosis of sharpshooters. Plos Biol 4:e188
Acknowledgments
We thank Dr. E. Virla for collecting D. maidis samples used in the present work and Dr. A Maciá for improving the English version of this manuscript. The authors especially thank Dr Hiroaki Noda for providing Nephotettix cincticeps specimens. This work was supported by FONCYT-PICT-2007-00143-03, Comisión de Investigaciones Científicas Pcia de Bs. As. (CIC), Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET), and Universidad Nacional de La Plata (UNLP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Handling Editor: Margit Pavelka
Rights and permissions
About this article
Cite this article
Brentassi, M.E., Franco, E., Balatti, P. et al. Bacteriomes of the corn leafhopper, Dalbulus maidis (DeLong & Wolcott, 1923) (Insecta, Hemiptera, Cicadellidae: Deltocephalinae) harbor Sulcia symbiont: molecular characterization, ultrastructure, and transovarial transmission. Protoplasma 254, 1421–1429 (2017). https://doi.org/10.1007/s00709-016-1033-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-016-1033-4