Summary.
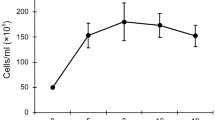
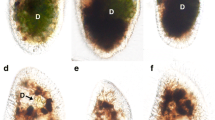
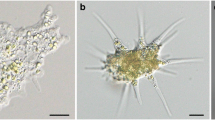
Paramecium bursaria cells harbor several hundred symbiotic algae in their cytoplasm. Algae-free cells can be reinfected with algae isolated from algae-bearing cells or cultivated Chlorella species through the digestive vacuoles. To determine the relationship between the infectivity of various Chlorella species and the nature of their cell wall components, algae-free P. bursaria cells were mixed with 15 strains of cultivated Chlorella species and observed for the establishment of endosymbiosis at 1 h and 3 weeks after mixing. Only 2 free-living algal strains, C. sorokiniana C-212 and C. kessleri C-531, were maintained in the host cells, whereas free-living C. sorokiniana C-43, C. kessleri C-208, C. vulgaris C-27, C. ellipsoidea C-87 and C-542, C. saccharophila C-183 and C-169, C. fusca var. vacuolata C-104 and C-28, C. zofingiensis C-111, and C. protothecoides C-150 and C-206 and the cultivated symbiotic Chlorella sp. strain C-201 derived from Spongilla fluviatilis could not be maintained. These infection-incapable strains could escape from the host digestive vacuole but failed to localize beneath the host cell membrane and were eventually digested. Labeling of their cell walls with Alexa Fluor 488-conjugated wheat germ agglutinin, GS-II, or concanavalin A, with or without pretreatment with 0.4 N NaOH, showed no relationship between their infectivity and the stainability with these lectins. Our results indicate that the infectivity of Chlorella species for P. bursaria is not based on the sugar residues on their cell wall and on the alkali-insoluble part of the cell wall components, but on their ability to localize just beneath the host cell membrane after escaping from the host digestive vacuole.
Similar content being viewed by others
Abbreviations
- Con A:
-
concanavalin A
- DIC:
-
differential-interference contrast
- DV:
-
digestive vacuole
- PV:
-
perialgal vacuole
- WGA:
-
wheat germ agglutinin
References
AK Allen A Neuberger N Sharon (1973) ArticleTitleThe purification, composition and specificity of wheat-germ agglutinin Biochem J 131 155–162 Occurrence Handle4737292 Occurrence Handle1:CAS:528:DyaE3sXptlyqtQ%3D%3D
R Bomford (1965) ArticleTitleInfection of alga-free Paramecium bursaria with strains of Chlorella, Scenedesmus, and a yeast J Protozool 12 221–224 Occurrence Handle5859658 Occurrence Handle1:STN:280:CCmD1c7pslA%3D
S Dryl (1959) ArticleTitleAntigenic transformation in Paramecium aurelia after homologous antiserum treatment during autogamy and conjugation J Protozool 6 IssueIDSuppl 25
S Ebisu IJ Goldstein (1978) Bandeiraea simplicifolia lectin II V Ginsburg (Eds) Complex carbohydrates, part C. Methods in enzymology NumberInSeries50 Academic Press London 350–354 Occurrence Handle10.1016/0076-6879(78)50041-4
IJ Goldstein CM Reichert A Misaki (1974) ArticleTitleInteraction of concanavalin A with model substrates Ann N Y Acad Sci 234 283–296 Occurrence Handle4213192 Occurrence Handle10.1111/j.1749-6632.1974.tb53040.x Occurrence Handle1:CAS:528:DyaE2cXlsV2qsbs%3D
IJ Goldstein S Hammarstrom G Sundblad (1975) ArticleTitlePrecipitation and carbohydrate-binding specificity studies on wheat germ agglutinin Biochim Biophys Acta 405 53–61 Occurrence Handle1174568 Occurrence Handle1:CAS:528:DyaE2MXlsVGltrw%3D
FK Gu L Chen B Ni X Zhang (2002) ArticleTitleA comparative study on the electron microscopic enzymo-cytochemistry of Paramecium bursaria from light and dark cultures Eur J Protistol 38 267–278 Occurrence Handle10.1078/0932-4739-00875
K Hiwatashi (1968) ArticleTitleDetermination and inheritance of mating type in Paramecium caudatum Genetics 58 373–386 Occurrence Handle5662626 Occurrence Handle1:STN:280:CCeA38fotlU%3D
H Hosoya K Kimura S Matsuda M Kitaura T Takahashi T Kosaka (1995) ArticleTitleSymbiotic algae-free strains of the green paramecium Paramecium bursaria produced by herbicide paraquat Zool Sci 12 807–810 Occurrence Handle1:CAS:528:DyaK28XhsFSlsbo%3D
HS Jennings (1938) ArticleTitleSex reaction types and their interrelations in Paramecium bursaria. I and II. Clones collected from natural habitats Proc Natl Acad Sci USA 24 112–120 Occurrence Handle16588202 Occurrence Handle10.1073/pnas.24.3.112 Occurrence Handle1:STN:280:DC%2BD28zhvVyjsw%3D%3D
MW Karakashian (1975) ArticleTitleSymbiosis in Paramecium bursaria Symp Soc Exp Biol 29 145–173 Occurrence Handle785659
SJ Karakashian (1963) ArticleTitleGrowth of Paramecium bursaria as influenced by the presence of algal symbionts Physiol Zool 36 52–68
SJ Karakashian MW Karakashian (1965) ArticleTitleEvolution and symbiosis in the genus Chlorella and related algae Evolution 19 368–377 Occurrence Handle10.2307/2406447
SJ Karakashian MA Rudzinska (1981) ArticleTitleInhibition of lysosomal fusion with symbiont-containing vacuoles in Paramecium bursaria Exp Cell Res 131 387–393 Occurrence Handle7202538 Occurrence Handle10.1016/0014-4827(81)90242-1 Occurrence Handle1:STN:280:Bi6C3sfkvFI%3D
Y Kodama M Fujishima (2005) ArticleTitleSymbiotic Chlorella sp. of the ciliate Paramecium bursaria do not prevent acidification and lysosomal fusion of the host digestive vacuoles in infection Protoplasma 225 191–203 Occurrence Handle15997335 Occurrence Handle10.1007/s00709-005-0087-5
Y Kodama M Nakahara M Fujishima (2007) ArticleTitleSymbiotic alga Chlorella vulgaris of the ciliate Paramecium bursaria shows temporary resistance to host lysosomal enzymes during the early infection process Protoplasma 230 61–67 Occurrence Handle17111098 Occurrence Handle10.1007/s00709-006-0193-z Occurrence Handle1:CAS:528:DC%2BD2sXis1Klsbs%3D
PN Lyer KD Wilkinson LJ Goldstein (1976) ArticleTitleAn N-acetyl-D-glycosamine binding lectin from Bandeiraea simplicifolia seeds Arch Biochem Biophys 177 330–333 Occurrence Handle999292 Occurrence Handle10.1016/0003-9861(76)90444-6 Occurrence Handle1:STN:280:CSiD28vltV0%3D
R Meier W Wiessner (1989) ArticleTitleInfection of algae-free Paramecium bursaria with symbiotic Chlorella sp. isolated from green paramecia. II. A timed study J Cell Sci 93 571–579
N Nishihara T Takahashi T Kosaka H Hosoya (1996) ArticleTitleCharacterization of endosymbiotic algae in Paramecium bursaria Jpn J Protozool 29 35
S Ogata T Muramatsu A Kobata (1975) ArticleTitleFractionation of glycopeptides by affinity column chromatography on concanavalin A-sepharose J Biochem 78 687–696 Occurrence Handle1213987 Occurrence Handle1:CAS:528:DyaE28XhvVOk
R Pado (1965) ArticleTitleMutual relation of protozoans and symbiotic algae in Paramecium bursaria. I. The influence of light on the growth of symbionts Folia Biol 13 173–182 Occurrence Handle1:STN:280:CCmD2c7gsFA%3D
RD Poretz IJ Goldstein (1970) ArticleTitleAn examination of the topography of the saccharide binding sites of concanavalin A and of the forces involved in complexation Biochemistry 9 2890–2896 Occurrence Handle5459542 Occurrence Handle10.1021/bi00816a021 Occurrence Handle1:CAS:528:DyaE3cXks1alsrY%3D
W Reisser (1976) ArticleTitleDie stoffwechselphysiologischen Beziehungen zwischen Paramecium bursaria Ehrbg. und Chlorella spec. in der Paramecium bursaria-Symbiose. I. Der Stickstoff- und der Kohlenstoff-Stoffwechsel Arch Microbiol 107 357–360 Occurrence Handle1275643 Occurrence Handle10.1007/BF00425352 Occurrence Handle1:CAS:528:DyaE28XktFOjsro%3D
W Reisser A Radunz W Wiessner (1982) ArticleTitleParticipation of algal surface structures in the cell recognition process during infection of aposymbiotic Paramecium bursaria with symbiotic chlorellae Cytobios 33 39–50 Occurrence Handle7105840 Occurrence Handle1:STN:280:Bi2B28%2FpsFM%3D
A Ryter R Hellio (1980) ArticleTitleElectron-microscope study of Dictyostelium discoideum plasma membrane and its modifications during and after phagocytosis J Cell Sci 41 75–88 Occurrence Handle7364890 Occurrence Handle1:STN:280:Bi%2BC28%2FitVw%3D
H Takeda T Hirokawa (1978) ArticleTitleStudies on the cell wall of Chlorella. I. Quantitative changes in cell wall polysaccharides during the cell cycle of Chlorella ellipsoidea Plant Cell Physiol 19 591–598 Occurrence Handle1:CAS:528:DyaE1cXltVeqs7c%3D
H Takeda T Sekiguchi S Nunokawa I Usuki (1998) ArticleTitleSpecies-specificity of Chlorella for establishment of symbiotic association with Paramecium bursaria. Does infectivity depend upon sugar components of the cell wall? Eur J Protistol 34 133–137
DS Weis (1969) ArticleTitleRegulation of host and symbiont population size in Paramecium bursaria Experientia 15 664–666 Occurrence Handle10.1007/BF01896584
DS Weis (1980) Hypothesis: Free maltose and algal cell surface sugars are signals in the infection of Paramecium bursaria by algae W Schwemmler EA Schenk (Eds) Endosymbiosis and cell biology NumberInSeriesI Walter de Gruyter Berlin 105–112
DS Weis A Ayala (1979) ArticleTitleEffect of exposure period and algal concentration on the frequency of infection of aposymbiotic ciliates by symbiotic algae from Paramecium bursaria J Protozool 26 245–248
Wichterman (1948) ArticleTitleThe biological effects of X-rays on mating types and conjugation of Paramecium bursaria Biol Bull 93 201
K Yamamoto T Tsuji I Matsumoto T Osawa (1981) ArticleTitleStructural requirements for the binding of oligosaccharides and glycopeptides to immobilized wheat germ agglutinin Biochemistry 29 5894–5899 Occurrence Handle10.1021/bi00523a037
Author information
Authors and Affiliations
Corresponding author
Additional information
Correspondence and reprints: Environmental Science and Engineering, Graduate School of Science and Engineering, Yamaguchi University, Yoshida 1677-1, Yamaguchi 753-8512, Japan.
Rights and permissions
About this article
Cite this article
Kodama, Y., Fujishima, M. Infectivity of Chlorella species for the ciliate Paramecium bursaria is not based on sugar residues of their cell wall components, but on their ability to localize beneath the host cell membrane after escaping from the host digestive vacuole in the early infection process. Protoplasma 231, 55–63 (2007). https://doi.org/10.1007/s00709-006-0241-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-006-0241-8