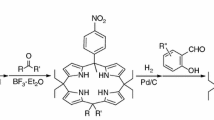
Abstract
We report the chemical synthesis and characterization of the stable 5,15-bis(pentafluorophenyl)-10-(trimethylsilylethynyl)corrole which serves as a precursor for the subsequent in situ sila-Sonogashira-cross-coupling reaction and metalation with copper(II) acetate. Under ambient conditions and a common catalyst system the reaction with 1-iodopyrene occurred within five hours. Due to the direct conjugation of the 18π-electronic system of the corrole macrocycle over the alkynyl group to the pyrene moiety the optical transitions in the Soret (B-) band Q-band region are significantly altered. The copper corrole exhibited complex hyperfine and superhyperfine structure in the EPR spectrum. The assignment of the EPR spectrum reveals the existence of an axial [CuII-cor∙+] species.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The establishment of novel synthesis routes towards symmetric and asymmetric corroles increased significantly over the past two decades [1,2,3,4,5]. This trend was triggered by the first published one-pot synthesis of corroles reported in 1999 by Gross and Paolesse [6, 7]. Since then, the scope of corrole application grew tremendously including examples in the fields of catalysis, photochemical sensors, molecular electronics, and biomedicinal applications [8,9,10,11,12,13]. For these purposes the availability of more sophisticated corrole systems is of great interest, which resulted in various contributions on corrole functionalization, including reactions on the β-pyrrolic positions such as bromination, hydroformylation, nitration, and chlorosulfonation as well as cycloadditions [3, 4]. Functionalizations on the meso-substituents include SNAr-reactions and Buchwald–Hartwig type Pd-catalyzed aminations, and Pd-catalyzed C–C cross-coupling reactions (Suzuki–Miyaura and Liebeskind–Srogl) on metallated (Cu, Ag, Mn) corroles have also been reported [12, 14,15,16,17,18]. To sum up, a broad variety of synthesis protocols to obtain meso-substituted A2B and A3-corroles exist and are well applicable [4, 5, 16, 19]. The aromatic substituents at the meso-positions of the corrole macrocycle normally twist out of planarity with respect to the 18 π-electron macrocycle and consequently an extended π-electron conjugation to the meso-substituent is interrupted. Alkynyl groups as linker between the corrole core and the aromatic systems could prevent this behavior and would enlarge the π-electron conjugation. Recently, Osuka et al. presented a new methodology to synthesize meso-cumulenic 2H-corroles from a meso-ethynyl-3H-corrole as precursor [20].
In the underlying research work we discuss the chemical synthesis and functionalization of meso-trimethylsilyl-ethynyl-3H-corrole via in situ sila-Sonogashira cross-coupling reactions to extend the π-electron conjugation from the 18π-electron macrocycle over the alkynyl group to adjacent electron rich systems.
Results and discussion
In this work, TMS-propynal was preferred as aldehyde for the corrole syntheses due to the high reactivity of propynal with nucleophiles and its tendency to form Michael addition products [21]. The first reaction step was the synthesis of 5-(pentafluoro)dipyrromethane (1) as precursor for the A2B-corrole synthesis. 1 was prepared according to the procedure of Dehean et al. [22] via condensation of pyrrole and pentafluorobenzaldehyde (Scheme 1) in water/HCl to give 89% yield. A prerequisite for further successful conversions is that pentafluorodipyrromethane 1 has to be purified via column chromatography and no other condensation product (tripyrromethane, bilane) must not be abundant.


With the dipyrromethane precursor in hand we started the A2B corrole synthesis according to the water/MeOH method published by Gryko and Koszarna (Scheme 2)[23]. The important factor of a biphasic system is that small starting materials are well soluble in this medium, wherein long chain pyrrole products such as bilane precipitate and so undesired reactions with different pyrrole derivatives are suppressed [23, 24]. 2 Eq. of the dipyrromethane precursor 1 and 1 eq. of TMS-propynal were suspended in the biphasic water/MeOH system with 3 mol% HCl as catalyst. After complete consumption of the educts the mixture was extracted thrice with 20 cm3 DCM. The solution, which contains bilane and longer chain pyrrole products, was evaporated to dryness, dissolved with 40 cm3 DCM and subsequently treated with 1.25 eq. of DDQ. The oxidation reaction was performed at room temperature and quenched after 15 min by immediate evaporation of the solvent. The crude solid was purified via silica column chromatography (for further details—please see the “Experimental” section). Unfortunately the desired A2B-corrole was not obtained, instead H3TpFPC (5,10,15-tris(pentafluorophenyl)corrole) resulted preferred. This side reaction can be explained due to an rearrangement of adjacent pyrrole moieties via the published acidolysis mechanism by Lindsey et al., which is called “scrambling” [25, 26]. A proposed mechanism is shown in Scheme 3. In the aqueous acidic milieu the dipyrromethane A is protonated at one of the α-positions, of the pyrrolic system, and after electronic rearrangement, a pyrrole molecule is eliminated and B results. B combines with another dipyrromethane and forms tripyrromethane C, which can further react with another molecule of B to form bilane D. After oxidation with DDQ, H3TpFPC 3 is generated.

In contrast to our results, the group of Gryko synthesized A2B corroles without scrambling and high yields up to 54% [23]. The characteristic difference is, that they used moderate till high reactive aromatic aldehydes, instead of the non-aromatic TMS-propynal.
In 2015 Gryko et al. published a paper [24], where A2B meso-arylethynylporphyrins were synthesized via a biphasic system, which inspired us to try water/THF as solvent mixture. 5-(Pentafluorophenyl)dipyrromethane and TMS-propynal were suspended, HCl as catalyst added and the reaction mixture stirred for 2.5 h at room temperature. After extraction with DCM the solution was concentrated to 20 cm3 and DDQ was added. The oxidation was stopped after 15 min and purification via silica column chromatography followed. The best conditions obtained via screening the reaction was a solvent ratio of 2:1 (THF/water) and 0.37 eq. HCl as catalyst (Table 1). The water/co-solvent ratio is a key factor, since with higher water content organic intermediate derivatives are less soluble and precipitate preferred [24]. Due to this reason, we performed syntheses with a higher water/THF ratio (Table 1) with the expectation of preferred product 2 formation. Instead of higher yields, the opposite occurred and only traces of 2 could be obtained. There is no evident trend in the performed screening series, related to HCl concentration or water/THF ratio.
We tried to synthesize the A2B-corrole 2 using trifluoroactetic acid (TFA) as another Brönsted acid according to the synthetic procedure of Gryko [26]. Following, the same equivalents of dipyrromethane and aldehyde, DCM instead of the biphasic system discussed above and variation of the TFA concentration resulted in the same result and only H3TpFPC 3 was obtained (Table 2). This led us to diversify the temperature, using the same mol% TFA as described in the literature [27], where best yields were achieved at − 20 °C (Table 2). We accomplished the successful synthesis of desired pure A2B-corrole 2 within 15 min reaction time during the condensation step and further oxidation at room temperature with a reaction yield of 10.1%. Prolonging the reaction time yielded in the formation of 3 and other side-products. At − 78 °C no further improvement of the reaction yield was obtained.
Inspired by the published synthetic procedure of Shaikh et al. [28], for a solvent free synthesis of dipyrromethanes with SnCl2·2H2O as catalyst, in the next step, we decided to use this method to synthesize corrole 2 with a series of Lewis acid catalysts.
First we varied the loading of the catalyst from equimolar to 0.04 equivalents with respect to the TMS-propynal. 0.64 mmol of DPM, 0.32 mmol TMS-propynal, and SnCl2·2H2O (0.04–1.0 eq.) were stirred in a 100 cm3 round-flask at room temperature for 10 min (Table 3). A black powder was formed, which was further dissolved in 7.5 cm3 DCM, 2.8 eq. DDQ were added portion wise and the solution was allowed to stir for 30 min. After common work-up the highest yield could be achieved using 0.04 eq. (Table 3) of the catalyst. With rising catalyst loadings the formation of H3TpFPC 3 is preferred, in contrast to the desired product 2. Entries 3–6 (Table 3) show four more Lewis acids which were investigated, due to their catalytic properties for the proper activation of the aldehyde. However, the syntheses were without success and again only H3TpFPC 3 was formed (Table 4).
In contrast to the results of Gryko et al. [19] and Osuka et al. [20] the deprotection of 2 occurred within few minutes with 1.5 eq. of TBAF (Scheme 4) and was determined via mass spectroscopy and UV–Vis measurements (see electronic supporting information). The isolation of the desilylated corrole 3 was more problematic due to its behaviour to decompose, which could be observed in 1H NMR in which signals in the range of 6–7 ppm were detected stemming from open-chain structure(s).

Consequently, further functionalisation of the corrole had to be done via a one-pot synthesis procedure starting from corrole 2. In this endeavour we decided to expand the π-system using sila-Sonogashira cross-coupling conditions to ensure decomposition of the corrole precursor would not occur (Scheme 5). Hence, 1 eq. of 2 was mixed with 1.5 eq. TBAF, 1 eq. 1-iodopyrene, 0.1 eq. Pd2dba3, 0.1 eq. CuI, and 1 eq. PPh3 in TEA/DCM (1:2) and was allowed to stir until no deprotected corrole 3 was observed in mass spectrum (Fig. SI and Fig. 1). Within 5 h reaction time the same reaction solution was treat with Cu(OAc)2 and after conventional work-up the corrole 5 was obtained with 57% yield.

a UV–Vis spectral and b mass spectrometric analysis during the one-pot sila-Sonogoshira of 2 (black line) to compound 4 (green line) and subsequent metalation with Cu(OAc)2 at room-temperature to the copper corrole 5 (blue line). c, d EPR spectra of 5 in chloroform at 300 K and 100 K. Simulated spectrum (inset, d)
Figure 1a, b exhibits the UV–Vis spectral and mass spectral changes during the reaction of corrole 2 via the desilylation process leading to corrole 3 and the subsequent conversion to the free-base 5,15-bis(pentafluorophenyl)-10-(pyreneethynyl)corrole 4 and finally the metalation with Cu(OAc)2 to the product 5. Strong shifts and splitting of the Soret band maxima of free-base 5,15-bis(pentafluorophenyl)-10-(pyreneethynyl)corrole 4 and the according copper corrole 5 are visible and suggest the strong coupling of the π-electron system of pyrene over the triple-bond to the 18 π-electronic system of the corrole. Moreover, the rather broad lines in the 1H and 19F NMR spectra (see Fig. S9 and Fig. S11) indicate at least partial contribution of d 9-species with Cu(II)corrole radical cation character, which can adopt either an antiferromagnetically coupled singlet state (saddle-shape geometry) or a planar triplet state [29]. Variable temperature EPR spectra of 5 were recorded in chloroform at 300 and 100 K and the corresponding spectra are shown in Fig. 1c, d, respectively. A square planar CuIII complex would be diamagnetic in nature while a CuII corrole should show an axial doublet for a single unpaired electron (S = 1/2) with hyperfine coupling of 65Cu/63Cu (I = 3/2) to give a four line spectrum. However, this signal is then further split by 14N (I = 1) to give a nine line spectral pattern. Indeed, defined EPR signals are observed for 5 with metal hyperfine (< 3100 Gauss) and ligand super-hyperfine interactions at low temperature, which accounts for the existence of [CuII-cor∙+] with the expected g∥ (or g z) = 2.173, g⊥ (or g x,y ) = 2.07, and A∥ = 19.3 mT (= 541 MHz) as shown in Fig. 1c, d (simulated spectrum illustrated as an insert in Fig. 1d) and, therefore, the non-innocence character of the corrole macrocycle. Electrochemical and spectrochemical investigations are in progress and beyond the scope of the underlying work.
Conclusion
We have established a novel synthesis procedure to obtain the 5,15-bis(pentafluorophenyl)-10-(trimethylsilylethynyl)corrole. This meso-substituted A2B corrole could not be synthesized via a standard procedure due to the competing fast scrambling process during the reaction. Successful conversion of the less reactive TMS-propynal (compared to the commonly employed arylaldehydes) with the dipyrromethane to the corrole was only obtained by the following the reaction conditions: 2 eq. DPM, 1 eq. TMS-propynal, and 0.5 mol% TFA (based on DPM) at − 20 °C (synthetic procedure A). The desired product was obtained in 10% yield. The stable 5,15-bis(pentafluorophenyl)-10-(trimethylsilylethynyl)corrole serves as a precursor for the subsequent functionalization via sila-Sonogashira-cross-coupling reaction with 1-iodopyrene under ambient conditions and a common catalyst system and further metalation with Cu(OAc)2. This functionalization procedure leads to an A2B corrole with an extended π-electronic structure at meso-position 10. The obtained free-base and copper corroles reveal strong changes in the UV–Vis spectral region.
Experimental
All chemicals were purchased from Alfa Aesar, Fluka, Merck, or Sigma-Aldrich and used without further purification. THF was distilled over sodium and benzophenone under an argon atmosphere and stored over molecular sieve (4 Å) until use. DCM was distilled over P2O5 under an argon atmosphere and stored over molecular sieve (4 Å) upon use. All solvents for the NMR were purchased from Euriso-Top. TLC was performed on Macherey–Nagel silica gel 60 (0.20 mm) with fluorescent indicator UV254 on aluminium plates and on Merck aluminium oxide 60 (0.20 mm) with fluorescent indicator UV254 on aluminium plates. For chromatography, silicagel columns were prepared with silicagel 60 (0.070–0.20 mesh) from Grace and aluminium oxide columns were prepared with aluminium oxide (activated, basic, Brockmann I) from Sigma-Aldrich. Proton (1H NMR) and Carbon (13C NMR) spectrum were recorded on a Bruker Ascend 700 MHz Avance III NMR spectrometer and on a Bruker Avance 300 MHz NMR spectrometer. Fluor (19F NMR) spectra were recorded on a Bruker Avance 300 MHz NMR spectrometer at 282.4 MHz. The chemical shifts are given in parts per million (ppm) on the delta scale (δ) and are referred to the used deuterated solvent for 1H NMR and to TFA for 19F NMR. Mass spectra were measured on a Finnigan LCQ DecaXPplus Ion trap mass spectrometer with ESI ion source and HRMS was performed on a 6510 quadrupole/time-of-flight (Q-TOF) instrument (Agilent). UV–Vis absorption spectra were collected on a Varian CARY 300 Bio spectrophotometer from 200 to 900 nm. Fluorescence spectra were measured on a CARY eclipse fluorescence spectrophotometer.
5-(Pentafluorophenyl)dipyrromethane (1)
Synthesis according to the procedure of Dehean et al. [22], 2.12 cm3 of freshly distilled pyrrole (30.6 mmol, 3 eq.) and 2.00 g of pentafluorobenzaldehyde (10.2 mmol, 1 eq.) were mixed with 100 cm3 0.18 M HCl and stirred for 5 h at room temperature under N2. After 5 h the precipitated product was filtered off and washed with water and heptane. Yield: 2.46 g (7.8 mmol, 77%); 1H NMR (300 MHz, CDCl3, 25 °C): δ = 8.20 (bs, 2H, NH), 6.75 (m, 2H, pyrrole-H), 6.19 (m, 2H, pyrrole-H), 6.05 (m, 2H, pyrrole-H), 5.92 (bs, 1H, meso-H) ppm; 19F NMR (300 MHz, CDCl3, 25 °C): δ = − 141.48 to − 141.42 (d, J(F,F) = 18 Hz, 2F, ortho-F), − 155.66 to − 155.81 (t, J(F,F) = 21 Hz, 1F, para-F), − 161.20 (m, 2F, meta-F) ppm; MS(ESI+): m/z calcd. for C15H9F5N2 312.07, found 313.0 ([M+H]+).
5,15-Bis(pentafluorophenyl)-10-(trimethylsilylethynyl)corrole (2, C36H20F10N4Si)
Method A: 47.3 mm3 of TMS-propynal (0.32 mmol, 1 eq.) and 100 mm3 of the TFA-solution (2.4 mm3 TFA in 1 cm3 CH2Cl2) were stirred under N2 atmosphere for 5 min in 2.0 cm3 CH2Cl2 at − 20 °C. Dropwise addition of 200 mg of 1 (0.64 mmol, 2 eq.) dissolved in 2.7 cm3 CH2Cl2 over 5 min followed. The reaction mixture was then stirred for 15 min at − 20 °C under N2 atmosphere. After dilution with CH2Cl2 to 7.2 cm3 and portion wise addition of 94.51 mg DDQ (0.42 mmol, 1.3 eq.), the solution was stirred for 20 min at room temperature under N2 atmosphere. Evaporation of the solvent and purification via column chromatography (silica, CH2Cl2/heptane 1:1) followed up. All fluorescent bands were collected and the solvent was evaporated to dryness. Yield: 23.5 mg (0.032 mmol, 10.1%).
Method B: A suspension containing 29.6 mm3 of TMS-propynal (0.20 mmol, 1 eq.), 2.5 cm3 THF, 1.25 cm3 H2O, and 0.6 mm3 HClconc was stirred under Ar atmosphere for 5 min at room temperature. Portion wise addition of 125 mg of 1 (0.40 mmol, 2 eq.) followed. The solution was stirred for 3 h under Ar atmosphere at room temperature. The mixture was extracted with CH2Cl2, the organic phase dried over Na2SO4 and concentrated. The collected brownish solid was dissolved in 45 cm3 CH2Cl2 and 59 mg DDQ (0.26 mmol, 1.3 eq.) added. After 30 min stirring at room temperature the solution was concentrated and purification via column chromatography (silica, CH2Cl2/heptane 1:1) followed. All fluorescent bands were collected and the solvent was evaporated to dryness. Yield: 8.2 mg (0.011 mmol, 5.7%).
Method C: 23.67 mm3 TMS-propynal (0.16 mmol, 1 eq.), 100 mg of 1 (0.32 mmol, 2 eq.), and 0.04 eq. of the Lewis acid were combined and stirred for 15 min at room temperature. Throughout the stirring the viscous mixture turned into a black powder, which was further dissolved in 7.5 cm3 DCM. DDQ (2.8 eq.) was added portion wise and the solution was allowed to stir at room temperature. After 30 min stirring at room temperature the solution was concentrated and purification via column chromatography (silica, CH2Cl2/heptane 1:1) followed. All fluorescent bands were collected and the solvent was evaporated to dryness. Yield: 2.0 mg (0.003 mmol, 1.7%).
1H NMR (300 MHz, CDCl3, 25 °C): δ = 9.38–9.40 (d, J(H,H) = 4.8 Hz, 2H, pyrrole-H), 9.00–9.02 (d, J(H,H) = 4.3 Hz, 2H, pyrrole-H), 8.71–8.73 (d, J(H,H) = 4.8 Hz, 2H, pyrrole-H), 8.47–8.48 (d, J(H,H) = 4.1 Hz, 2H, pyrrole-H), 0.59 (bs, 9H, CH3) ppm; 19F NMR (300 MHz, CDCl3, 25 °C): δ = −141.48 to − 141.42 (d, J(F,F) = 18 Hz, 2F, ortho-F), − 155.66 to − 155.81 (t, J(F,F) = 21 Hz, 1F, para-F), -161.20 (m, 2F, meta-F) ppm; MS(ESI−): m/z calcd. for C36H20F10N4Si ([M-H]+) 725.1209, found 727.1219; UV/Vis (CH2Cl2): λ max (ε) = 427 (7.93 × 103), 575 (1.85 × 103), 624 (1.37 × 103) nm (dm3 mol−1 cm−1).
Copper 5,15-bis(pentafluorophenyl)-10-(pyreneethynyl)corrole (5, C49H17CuF10N4)
10 mg TMS-ethynylcorrole 1 (0.013 mmol) was dissolved in 3.2 cm3 DCM and 1.9 cm3 TEA. The solution was purged with argon and several freeze–pump–thaw cycles followed. 5.3 mg TBAF (0.020 mmol, 1.5 eq.), 4.5 mg Pd2dba3 (0.013 mmol, 1 eq.), 10 mol% CuI, and 10 mol% PPh3 were added and the resulting mixture was stirred at room temperature under argon overnight. After completion of the reaction, 7 mg Cu(OAc)2 (0.039 mmol, 3.3 eq.) were added the reaction was allowed to stir for another hour. The crude was filtered through Celite and then washed by extraction with water and dichloromethane. The organic phase was dried over Na2SO4 and concentrated. Purification was accomplished via column chromatography (silica, CH2Cl2/heptane 5:1). Yield: 7.1 mg (0.008 mmol, 57%). 1H NMR (300 MHz, CDCl3, 25 °C): δ = 8.49 (bs, 1H, pyrrole-H), 8.28 (bs, 1H, pyrrole-H), 8.19 (m, 2H, pyrrole-H), 8.13 (m, 1H, pyrrole-H), 8.01 (bs, 2H, pyrrole-H), 7.85 (bs, 1H, pyrrole-H), 7.71 (bs, 1H, pyrene-H), 7.61 (bs, 4H, pyrene-H), 7.53 (bs, 1H, pyrene-H), 7.44 (bs, 1H, pyrene-H), 7.40 (bs, 2H, pyrene-H) ppm; 19F NMR (300 MHz, CDCl3, 25 °C): δ = -137.67 (m, 4F, ortho-F), -152.22 (m, 2F, para-F), -161.43 (m, 4F, meta-F) ppm; MS(ESI−): m/z calcd. for C49H17F10N4Cu 914.0590, found 914.0549 ([M−H]+); UV/Vis (CH2Cl2): λ max (ε) = 269 (5.66 × 103), 280 (6.76 × 103), 319 (5.53 × 103), 334 (7.02 × 103), 351 (7.09 × 103), 409 (7.39 × 103), 426 (6.85 × 103), 464 (5.41 × 103), 570 (2.74 × 103), 609 (2.33 × 103), 645 (1.88 × 103) nm (dm3 mol−1 cm−1).
References
Lemon CM, Brothers PJ (2011) J Porphyrins Phthalocyanines 15:809
Paolesse R (2008) Synlett 15:2215
Barata JFB, Neves MGPMS, Faustino MAF, Tomé AC, Cavaleiro JAS (2017) Chem Rev 117:3192
Orłowski R, Gryko D, Gryko DT (2017) Chem Rev 117:3102
König M, Faschinger F, Reith LM, Schöfberger W (2016) J Porphyrins Phthalocyanines 20:96
Paolesse R, Mini S, Sagone F, Boschi T, Jaquinod L, Nurco DJ, Smith KM (1999) Chem Commun 14:1307
Gross Z, Galili N (1999) Angew Chem Int Ed 111:2536
Borisov SM, Alemayehu A, Ghosh A (2016) J Mater Chem C 4:5822
Paolesse R, Nardis S, Monti D, Stefanelli M, Di Natale C (2017) Chem Rev 117:2517
Lemon CM, Powers DC, Brothers PJ, Nocera DG (2017) Inorg Chem 56:10991
Aviv-Harel I, Gross Z (2009) Chem Eur J 15:8382
Schöfberger W, Faschinger F, Chattopadhyay S, Bhakta S, Mondal B, Elemans JAAW, Müllegger S, Tebi S, Koch R, Klappenberger F, Paszkiewicz M, Barth JV, Rauls E, Aldahhak H, Schmidt WG, Dey A (2016) Angew Chem Int Ed 55:2350
Teo RD, Hwang JY, Termini J, Gross Z, Gray HB (2017) Chem Rev 117:2711
Stefanelli M, Mastroianni M, Nardis S, Licoccia S, Fronczek FR, Smith KM, Zhu W, Ou Z, Kadish KM, Paolesse R (2007) Inorg Chem 46:10791
Schmidlehner M, Faschinger F, Reith LM, Ertl M, Schoefberger W (2013) Appl Organomet Chem 27:395
König M, Reith LM, Monkowius U, Knör G, Bretterbauer K, Schoefberger W (2011) Tetrahedron 67:4243
Tiffner M, Gonglach S, Haas M, Schöfberger W, Waser M (2017) Chem Asian J 12:1048
Sinha W, Sommer MG, Deibel N, Ehret F, Sarkar B, Kar S (2014) Chem Eur J 20:15920
Gryko DT (2002) Eur J Org Chem 11:1735
Ueta K, Naoda K, Ooi S, Tanaka T, Osuka A (2017) Angew Chem Int Ed 56:7223
Anderson HL (1992) Tetrahedron Lett 33:1101
Rohand T, Dolusic E, Ngo T, Maes W, Dehean W (2007) Arkivoc Part(x):307
Koszarna B, Gryko DT (2006) J Org Chem 71:3707
Nowak-Król A, Plamont R, Canard G, Edzang JA, Gryko DT, Balaban TS (2015) Chem Eur J 21:1488
Littler BJ, Ciringh Y, Lindsey JS (1999) J Org Chem 64:2864
Geier GR III, Littler BJ, Lindsey JS (2001) J Chem Soc Perkin Trans 2:701
Gryko DT, Jadach K (2001) J Org Chem 66:4267
Shaikh KA, Patil VA, Bandgar BP (2012) Orbital Electron J Chem 4:111
Yadav P, Sankar M, Ke X, Cong L, Kadish KM (2017) Dalton Trans 46:10014
Acknowledgements
Open access funding provided by Austrian Science Fund (FWF). We acknowledge the financial support of the Austrian Science Fund (FWF- P28167-N34). The NMR spectrometers were acquired in collaboration with the University of South Bohemia (CZ) with financial support from the European Union through the EFRE INTERREG IV ETC-AT-CZ program (Project M00146, “RERI-uasb”). We would like to thank Dr. Markus Himmelsbach for the measurement of ESI–MS spectra.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Haas, M., Gonglach, S., Müllegger, S. et al. Synthesis and characterization of meso-substituted A2B corroles with extended π-electronic structure. Monatsh Chem 149, 773–781 (2018). https://doi.org/10.1007/s00706-017-2114-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-017-2114-6