Abstract
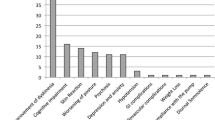
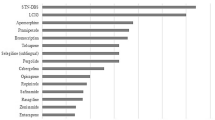
Development of motor fluctuations and dyskinesia characterizes the transition from early to advanced Parkinson disease stage. Current therapeutic strategies to manage motor complications aim at increasing the number of levodopa administrations and extending its benefit by the association of enzyme blockers and dopamine agonists. However, as disease progresses, mobility becomes progressively dependent on levodopa absorption and its plasma bioavailability, resulting in loss of independence, worse quality of life and increased caregiver burden. If patients continue to experience off-time with functional impact on activities of daily living after best medication adjustments, implementation of infusion with apomorphine or levodopa, and surgical therapies should be considered. Presence of troublesome dyskinesia would also favor the choice of an advanced treatment. Compared with pulsatile oral therapy, both apomorphine and levodopa infusion determine more continuous striatal dopamine receptors stimulation than oral levodopa resulting in significant reduction of off-time and dyskinesia, particularly peak-dose, although not in their complete resolution. This observation proves that abnormal synaptic plasticity and connectivity changes cannot be reversed once they are established. Early implementation of these therapeutic strategies ideally would target patients as soon as motor complications begin rather than at late stage of advanced Parkinson’s disease (PD) before dyskinesia have manifested. Preliminary evidence from early deep brain stimulation in patients with short disease duration and modest motor complications suggests that this approach can positively impact quality of life. It is conceivable that changing our PD treatment algorithm and implementing device-aided therapies at the beginning of the advanced phase before dyskinesia has established, will provide more stable motor conditions and longer functional autonomy.

Similar content being viewed by others
References
Antonini A, Tolosa E (2009) Apomorphine and levodopa infusion therapies for advanced Parkinson’s disease: selection criteria and patient management. Expert Rev Neurother 9:859–867
Antonini A, Chaudhuri KR, Martinez-Martin P, Odin P (2010) Oral and infusion levodopa-based strategies for managing motor complications in patients with Parkinson’s disease. CNS Drugs 24:119–129
Antonini A, Isaias IU, Rodolfi G et al (2011) A 5-year prospective assessment of advanced Parkinson disease patients treated with subcutaneous apomorphine infusion or deep brain stimulation. J Neurol 258:579–585
Antonini A, Odin P, Lopiano L et al (2013) Effect and safety of duodenal levodopa infusion in advanced Parkinson’s disease: a retrospective multicenter outcome assessment in patient routine care. J Neural Transm 120:1553–1558
Antonini A, Fung VS, Boyd JT et al (2016) Effect of levodopa-carbidopa intestinal gel on dyskinesia in advanced Parkinson’s disease patients. Mov Disord 31:530–537
Antonini A, Poewe W, Chaudhuri KR, GLORIA Study Co-investigators et al (2017) Levodopa-carbidopa intestinal gel in advanced Parkinson’s: final results of the GLORIA registry. Parkinsonism Relat Disord 45:13–20
Antonini A, Moro E, Godeiro C, Reichmann H (2018a) Medical and surgical management of advanced Parkinson’s disease. Mov Disord. https://doi.org/10.1002/mds.27340
Antonini A, Robieson WZ, Bergmann L, Yegin A, Poewe W (2018b) Age/disease duration influence on activities of daily living and quality of life after levodopa-carbidopa intestinal gel in Parkinson’s disease. Neurodegener Dis Manag 8:161–170
Bellucci A, Antonini A, Pizzi M, Spano P (2017) The end is the beginning: Parkinson’s disease in the light of brain imaging. Front Aging Neurosci 9:330. https://doi.org/10.3389/fnagi.2017.00330
Bezard E (2013) Experimental reappraisal of continuous dopaminergic stimulation against l-dopa-induced dyskinesia. Mov Disord 28:1021–1022
Bjornestad A, Forsaa EB, Pedersen KF, Tysnes OB, Larsen JP, Alves G (2016) Risk and course of major complications in a population-based incident Parkinson’s disease cohort. Park Relat Disord 22:48–53
Borgemeester RW, Drent M, van Laar T (2016) Motor and non-motor outcomes of continuous apomorphine infusion in 125 Parkinson’s disease patients. Park Relat Disord 23:17–22
Cabrera LY, Goudreau J, Sidiropoulos C (2018) Critical appraisal of the recent US FDA approval for earlier DBS intervention. Neurology. https://doi.org/10.1212/WNL.0000000000005829
Calabresi P, Di Filippo M, Ghiglieri V, Tambasco N, Picconi B (2010) Levodopa-induced dyskinesias in patients with Parkinson’s disease: filling the bench-to-bedside gap. Lancet Neurol 9(11):1106–1117
Carta M, Bezard E (2011) Contribution of pre-synaptic mechanisms to l-DOPA-induced dyskinesia. Neuroscience 198:245–251
Cenci MA, Crossman AR (2018) Animal models of l-dopa-induced dyskinesia in Parkinson’s disease. Mov Disord. https://doi.org/10.1002/mds.27337
Coelho M, Ferreira JJ (2012) Late-stage Parkinson disease. Nat Rev Neurol 8:435–442
Dafsari HS, Reker P, Silverdale M et al (2017) Subthalamic stimulation improves quality of life of patients aged 61 years or older with short duration of Parkinson’s disease. Neuromodulation. https://doi.org/10.1111/ner.12740
Drapier S, Eusebio A, Degos B et al (2016) Quality of life in Parkinson’s disease improved by apomorphine pump: the OPTIPUMP cohort study. J Neurol 263:1111–1119
Elkurd MT, Bahroo LB, Pahwa R (2018) The role of extended-release amantadine for the treatment of dyskinesia in Parkinson’s disease patients. Neurodegener Dis Manag
Fabbri M, Coelho M, Guedes LC et al (2017) Response of non-motor symptoms to levodopa in late-stage Parkinson’s disease: results of a levodopa challenge test. Parkinsonism Relat Disord 39:37–43
Fahn S, Oakes D, Shdoulson I et al (2004) Levodopa and the progression of Parkinson’s disease. N Engl J Med 351:2498–2508
Fox SH, Katzenschlager R, Lim SY et al (2011) The movement disorder society evidence-based medicine review update: treatments for the motor symptoms of Parkinson’s disease. Mov Disord 26(Suppl 3):S2–S41
Hadj Tahar A, Gregoire L, Bangassoro E, Bedard PJ (2000) Sustained cabergoline treatment reverses levodopa-induced dyskinesias in parkinsonian monkeys. Clin Neuropharmacol 23:195–202
Jenner P, Katzenschlager R (2016a) Apomorphine—pharmacological properties and clinical trials in Parkinson’s disease. Parkinsonism Relat Disord 33(Suppl 1):S13–S21
Jenner P, Katzenschlager R (2016b) Apomorphine—pharmacological properties and clinical trials in Parkinson’s disease. Park Relat Disord 33(Suppl 1):S13–S21
Katzenschlager R, Hughes A, Evans A et al (2005) Continuous subcutaneous apomorphine therapy improves dyskinesias in Parkinson’s disease: a prospective study using single-dose challenges. Mov Disord 20:151–157
Katzenschlager R, Poewe W, Rascol O et al (2018) Double-blind, randomised, placebo-controlled, phase III study (Toledo) to evaluate the efficacy of apomorphine subcutaneous infusion in reducing ‘off’ time in Parkinson’s disease patients with motor fluctuations not well controlled on optimised medical treatment. Lancet Neurol (in press)
Lang AE, Rodriguez RL, Boyd JT et al (2016) Integrated safety of levodopa-carbidopa intestinal gel from prospective clinical trials. Mov Disord 31:538–546
Maratos EC, Jackson MJ, Pearce RK, Cannizzaro C, Jenner P (2003) Both short- and long-acting D-1/D-2 dopamine agonists induce less dyskinesia than l-DOPA in the MPTP-lesioned common marmoset (Marmoset/Callithrix jacchus). Exp Neurol 179:90–102
Martinez-Martin P, Reddy P, Katzenschlager R et al (2015) EuroInf: a multicenter comparative observational study of apomorphine and levodopa infusion in Parkinson’s disease. Mov Disord 30:510–516
Odin P, Ray Chaudhuri K, Slevin JT, National Steering Committees et al (2015) Collective physician perspectives on non-oral medication approaches for the management of clinically relevant unresolved issues in Parkinson’s disease: consensus from an international survey and discussion program. Parkinsonism Relat Disord 21:1133–1144
Olanow CW, Kieburtz K, Odin P et al (2014) Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol 13:141–149
Pilleri M, Antonini A (2015) Therapeutic strategies to prevent and manage dyskinesias in Parkinson’s disease. Expert Opin Drug Saf 14:281–294
Porras G, De Deurwaerdere P, Li Q, Marti M, Morgenstern R, Sohr R, Bezard E, Morari M, Meissner WG (2014) l-Dopa-induced dyskinesia: beyond an excessive dopamine tone in the striatum. Sci Rep 4:3730
Schuepbach WM, Rau J, Knudsen K et al (2013) Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med 368:610–622
Sensi M, Cossu G, Mancini F et al (2017) Which patients discontinue? Issues on Levodopa/carbidopa intestinal gel treatment: Italian multicentre survey of 905 patients with long-term follow-up. Parkinsonism Relat Disord 38:90–92
Sesar Á, Fernández-Pajarín G, Ares B, Rivas MT, Castro A (2017) Continuous subcutaneous apomorphine infusion in advanced Parkinson’s disease: 10-year experience with 230 patients. J Neurol 264:946–954
Sharma JC, Ross IN, Rascol O, Brooks D (2008) Relationship between weight, levodopa and dyskinesia: the significance of levodopa dose per kilogram body weight. Eur J Neurol 15:493–496
Stocchi F, Ruggieri S, Vacca L, Olanow CW (2002) Prospective randomized trial of lisuride infusion versus oral levodopa in patients with Parkinson’s disease. Brain 125:2058–2066
Timpka J, Fox T, Fox K, Honig H, Odin P, Martinez-Martin P, Antonini A, Chaudhuri KR (2016) Improvement of dyskinesias with l-dopa infusion in advanced Parkinson’s disease. Acta Neurol Scand 133:451–458
Timpka J, Nitu B, Datieva V, Odin P, Antonini A (2017) Device-aided treatment strategies in advanced Parkinson’s disease. Int Rev Neurobiol 132:453–474
Vizcarra JA, Situ-Kcomt M, Artusi CA et al (2018) Subthalamic deep brain stimulation and levodopa in Parkinson’s disease: a meta-analysis of combined effects. J Neurol. https://doi.org/10.1007/s00415-018-8936-2
Wirdefeldt K, Odin P, Nyholm D (2016) Levodopa-carbidopa intestinal gel in patients with Parkinson’s disease: a systematic review. CNS Drugs 30:381–404
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no competing interest.
Rights and permissions
About this article
Cite this article
Antonini, A., Nitu, B. Apomorphine and levodopa infusion for motor fluctuations and dyskinesia in advanced Parkinson disease. J Neural Transm 125, 1131–1135 (2018). https://doi.org/10.1007/s00702-018-1906-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-018-1906-0