Abstract
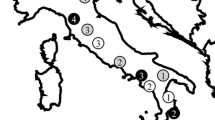
The orchid genus Ophrys operates a system of sexual deception by which high specificity of pollination is attained. Reproductive isolation in Ophrys mainly rests upon prezygotic isolation mechanisms. The level of genetic separateness of Ophrys taxa with different pollinators is therefore likely determined by the fidelity of pollinators. The present study employs genetic fingerprinting to investigate this in the east Aegean Ophrys omegaifera s.l. complex, also including O. dryis, a west Mediterranean species of this complex. Ophrys fleischmannii, O. basilissa, and the west Mediterranean O. dyris, are found to be well-separated genetic entities whereas O. omegaifera s.str. and the putative hybrid taxon, O. sitiaca, are found to be genetically inseparable across their entire range of co-occurrence. This suggests that specific pollinators have high enough fidelity to act as effective isolating factors in east Aegean O. omegaifera s.l. as a whole, but that the situation in the species pair of O. sitiaca and O. omegaifera is likely to be more complex.
Similar content being viewed by others
References
Alibertis C., Alibertis A. and Reinhard H. R. (1990). Untersuchungen am Ophrys omegaifera – Komplex Kretas. Mitt. Bl. Arbeitskr. Heim. Orch. Baden-Württ. 22: 181–236
Arditti J. and Ghani A. K. A. (2000). Tansely Review No. 110. Numerical and physical properties of orchid seeds and their biological implications. New Phytol. 145: 367–421
Bateman R. M., Hollingsworth P. M., Preston J., Luo J.-B., Pridgeon A. M. and Chase M. W. (2003). Molecular phylogenetics and evolution of Orchidinae and selected Habenariinae (Orchidaceae). Bot. J. Linn. Soc. 142: 1–40
Benham J. J., Jeung J.-U., Jasieniuk M. A., Kanazin V. and Blake T. K. (1999). Genographer: a graphical tool for automated fluorescent AFLP and microsatellite analysis. J. Agric. Genom. 4: 399
Bernardos S., Amich F. and Gallego F. (2003). Karyological and taxonomical notes on Ophrys (Orchidoideae, Orchidaceae) from the Iberian Peninsula. Bot. J. Linn. Soc. 142: 395–406
Bernardos S., Crespí A., del Rey F. and Amich F. (2005). The section Pseudophrys (Ophrys, Orchidaceae) in the Iberian Peninsula: a morphometric and molecular analysis. Bot. J. Linn. Soc. 148: 359–375
Bonin A., Bellemain E., Bronken Eidesen P., Pompanon F., Brochmann C. and Taberlet P. (2004). How to track and assess genotyping errors in population genetic studies. Molec. Ecol. 13: 3261–3273
Buerkle C. A. (2005). Maximum-likelihood estimation of a hybrid index based on molecular markers. Molec. Ecol. Notes 5: 684–687
Casgrain P., Legendre P. (2004) The R package for multivariate and spatial analysis, version 4.0 (development release 9). Department of Biological Sciences, University of Montreal, Montreal, Canada.
Corander J., Waldmann P. and Sillanpää M. J. (2003). Bayesian analysis of genetic differentiation between populations. Genetics 163: 367–374
Cozzolino S., D'Emerico S. and Widmer A. (2004). Evidence for reproductive isolate selection in Mediterranean orchids: karyotype differences compensate for the lack of pollinator specificity. Proc. Roy. Soc. Lond. B. 271: S259–S262
Delforge P. (2005). Guide des orchidées d'Europe, d'Afrique du Nord et du Proche-Orient, 3rd ed. Delachaux et Niestlé, Paris, France
D'Emerico S., Pignone D., Bartolo G., Pulvirenti S., Terrasi C., Stuto S. and Scrugli A. (2005). Karyomorphology, heterochromatin patterns and evolution in the genus Ophrys (Orchidaceae). Bot. J. Linn. Soc. 148: 87–99
Ehrendorfer F. (1980) Hybridisierung, Polyploidie und Evolution bei europäisch-mediterranen Orchideen. Orchidee Sonderheft: 15–34.
Gölz P., Reinhard H. R., Alibertis C., Alibertis A., Gack C. and Paulus H. F. (1996). Gestaltwandel innerhalb kretischer Orchideenaggregate im Verlauf der Monate Januar bis Mai. J. Eur. Orch. 28: 641–701
Grant V. (1994). Modes and origins of mechanical and ethological isolation in angiosperms. Proc. Natl. Acad. Sci. USA 91: 3–10
Greilhuber J. and Ehrendorfer F. (1975). Chromosome numbers and evolution in Ophrys (Orchidaceae). Pl. Syst. Evol. 124: 125–138
Hedrén M., Fay M. F. and Chase M. W. (2001). Amplified fragment length polymorphisms (AFLP) reveal details of polyploid evolution in Dactylorhiza (Orchidaceae). Amer. J. Bot. 88: 1868–1880
Huson D. H. and Bryant D. (2006). Application of phylogenetic networks in evolutionary studies. Molec. Biol. Evol. 23: 254–267
Jaccard P. (1908). Nouvelles recherches sur la distribution florale. Bull. Soc. Vaud. Sci. Nat. 44: 223–270
Kretzschmar H., Kretzschmar G. and Eccarius W. (2002). Orchideen auf Kreta, Kasos und Karpathos. Ein Feldführer durch die Orchideenflora der zentralen Insel der Südägäis. Selbstverlag H. Kretzschmar, Bad Hersfeld, Germany
Kullenberg B. (1961). Studies in Ophrys pollination. Zool. Bidr. Uppsala 34: 1–340
Mant J. G., Peakall R. and Schiestl F. P. (2005). Does selection on floral odor promote differentiation among populations and species of the sexually orchid genus Ophrys. Evolution 59: 1449–1463
Nei M. and Li W.-H. (1979). Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. USA 76: 5269–5273
Paulus H. F. (1988). Beobachtungen zur Pseudokopulation auf Ophrys-Arten (Orchidaceae) Kretas (II) – mit einer Beschreibung von Ophrys sitiaca H. F. Paulus & C. + A. Alibertis nov. spec. aus dem Ophrys fusca – omegaifera Formenkreis. Mitt. Bl. Arbeitskr. Heim. Orch. Baden-Württ. 20: 817–822
Paulus H. F. (2001). Material zu einer Revision des Ophrys fusca s.str. Artenkreises I. Ophrys nigroaenea-fusca, O. colletes-fusca, O. flavipes-fusca, O. funerea, O. forestieri oder was ist die typische Ophrys fusca Link 1799 (Orchidaceae)?. J. Eur. Orch. 33: 121–177
Paulus H. F. (2006). Deceived males - Pollination biology of the Mediterranean orchid genus Ophrys (Orchidaceae). J. Eur. Orch. 38: 303–353
Paulus H. F. and Gack C. (1981). Neue Beobachtungen zur Bestäubung von Ophrys (Orchidaceae) in Südspanien, mit besonderer Berücksichtigung des Formenkreises Ophrys fusca agg. Pl. Syst. Evol. 137: 241–358
Paulus H. F. and Gack C. (1990a). Pollination in Ophrys (Orchidaceae) in Cyprus. Pl. Syst. Evol. 169: 177–207
Paulus H. F. and Gack C. (1990b). Pollinators as prepollinating isolation factors: evolution and speciation in Ophrys (Orchidaceae). Israel J. Bot. 39: 43–79
Paulus H. F., Gack C. (1994) Signalfäschung als Bestäubungsstrategie in der mediterranen Orchideengattung Ophrys - Probleme der Artbildung und der Artabgrenzung. In: Brederoo P., Kapteyn den Boumeester D. W. (eds.) International Symposium of European Orchids, Euorchis 1992, Utrecht/Haarlem, pp. 45–71.
Pfosser M. F., Jakubowsky G., Schlüter P. M., Fer T., Kato H., Stuessy T. F. and Sun B.-Y. (2006). Evolution of Dystaenia takesimana (Apiaceae), endemic to Ullung Island, Korea. Pl. Syst. Evol. 256: 159–170
Podani J. (2001). SYN-TAX 2000. Computer programs for data analysis in ecology and systematics. Scientia Publishing, Budapest, Hungary
Pritchard J. K., Stephens M. and Donnelly P. (2000). Inference of population structure using multilocus genotype data. Genetics 155: 945–959
Schiestl F. P. and Ayasse M. (2002). Do changes in floral odor cause speciation in sexually deceptive orchids. Pl. Syst. Evol. 234: 111–119
Schiestl F. P., Ayasse M., Paulus H. F., Löfstedt C., Hansson B. S., Ibarra F. and Francke W. (1999). Orchid pollination by sexual swindle. Nature 399: 421–422
Schiestl F. P., Ayasse M., Paulus H. F., Löfstedt C., Hansson B. S., Ibarra F. and Francke W. (2000). Sex pheromone mimicry in the early spider orchid (Ophrys sphegodes): patterns of hydrocarbons as the key mechanism for pollination by sexual deception. J. Comp. Physiol. A 186: 567–574
Schlüter P. M. and Harris S. A. (2006). Analysis of multilocus fingerprinting data sets containing missing data. Molec. Ecol. Notes 6: 569–572
Schönswetter P., Tribsch A. and Niklfeld H. (2004). Amplified fragment length polymorphism (AFLP) reveals no genetic divergence of the Eastern Alpine endemic Oxytropis campestris subsp. tiroliensis (Fabaceae) from widespread subsp. campestris. Pl. Syst. Evol. 244: 245–255
Soliva M., Kocyan A. and Widmer A. (2001). Molecular phylogenetics of the sexually deceptive orchid genus Ophrys (Orchidaceae) based on nuclear and chloroplast DNA sequences. Molec. Phylogenet. Evol. 20: 78–88
Soliva M. and Widmer A. (2003). Gene flow across species boundaries in sympatric, sexually deceptive Ophrys (Orchidaceae) species. Evolution 57: 2252–2261
Stökl J., Paulus H. F., Dafni A., Schulz C., Francke W. and Ayasse M. (2005). Pollinator attracting odour signals in sexually deceptive orchids of the Ophrys fusca group. Pl. Syst. Evol. 254: 105–120
Swofford D. L. (2002). PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sinauer Associates, Sunderland, MA, USA
Tremetsberger K., Stuessy T. F., Guo Y.-P., Baeza C. M., Weiss-Schneeweiss H. and Samuel R. M. (2003). Amplified fragment length polymorphism (AFLP) variation within and among populations of Hypochaeris acaulis (Asteraceae) of Andean southern South America. Taxon 52: 237–245
Tyteca D., Benito Ayuso J. and Walravens M. (2003). Ophrys algarvensis, a new species from the southern Iberian Peninsula. J. Eur. Orch. 35: 57–78
Vos P., Hogers R., Bleeker M., Reijans M., van de Lee T., Hornes M., Frijters A., Pot J., Peleman J., Kuiper M. and Zabeau M. (1995). AFLP: a new technique for DNA fingerprinting. Nucl. Acids Res. 23: 4407–4414
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schlüter, P., Ruas, P., Kohl, G. et al. Reproductive isolation in the Aegean Ophrys omegaifera complex (Orchidaceae). Plant Syst. Evol. 267, 105–119 (2007). https://doi.org/10.1007/s00606-007-0557-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-007-0557-7