Abstract
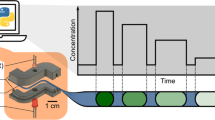
Opto-chemical capillary clocks are presented that are based on the measurement of a colored segment in a microchannel (a capillary). Color is created by a chromogenic chemistry involving the oxidation of a (virtually colorless) leuco-dye. Poly(ethylene glycol) (PEG) is used as a solvent, and indigo and thioindigo (in their reduced leuco forms) act as oxygen-sensitive dyes. The clock is started by removing one seal at the end of the capillary. A visible color change occurs as air diffuses into the microchannel due to an irreversible color reaction. The length of the colored segment is proportional to the time elapsed. PEGs of different average molar mass affect the diffusion rate of oxygen in the microchannel and thereby affect the rate of the migration of the color front. Both temperature and relative humidity exert a strong effect. Six types of such clocks are described that enable times to be determined in the range from 1 day to 6 months, possibly of even decades.

Optical clocks are presented where elapsed time is indicated by the length of the colored segment of a micro-capillary.






Similar content being viewed by others
References
Galagan Y, Su WF (2008) Fadable ink for time-temperature control of food freshness: novel new time-temperature indicator. Food Res Int 41:653–657
Galagan Y, Hsu SH, Su WF (2009) Monitoring time and temperature by methylene blue containg polyacrylate film. Sens Actuators B: Chem 144:49–55
Bommarito GM, Mazurek MH, Johnston RP, Yarusso DJ, Larson CL (2004) Microstructured time dependent indicators. US Patent 6,741,523 B1
Hsu YC, Sair AI, Booren AM, Smith DM (2000) Triose phosphate isomerase as an endogenous time-temperature integrator to verify adequacy of roast beef processing. J Food Sci 65:236–240
Mendoza TF, Welt BA, Otwell S, Teixeira AA, Kristonsson H, Balaban MO (2004) Kinetic parameter estimation of time-temperature integrators intended for use with packaged fresh seafood. J Food Sci 69:FMS90–FMS96
Ocio MJ, Fernandez PS, Rodrigo M, Periago P, Martinez A (1997) A time-temperature integrator for particulated food: thermal process evaluation. Z Lebensm Unters Forsch A 205:325–328
Rodrigo F, Rodrigo MC, Martinez A (1998) Evaluation of a new time temperature integrator in pilot plant conditions. Z Lebensm Unters Forsch A 206:184–188
Arens RP, Birkholz RD, Johnson DL, Labuza TP, Larson CL, Yarusso DJ (1997) Time-temperature integrating indicator device. US Patent 5,667,303
Haas DJ, Holt RJ, Davis LH (2006) Long term rapid color changing time indicator. US Patent 7,139,226 B2
Haas DJ (1990) Time indicator enhancement method. US Patent 4,903,254
Haas DJ, Haas SF (1997) Long term rapid color changing time indicator. US Patent 5,633,835
Manske WJ (1976) Selected time interval indicating device. US Patent 3,954,011
Jackson D (1969) Time indicator. US Patent 3,480,402
Robertson GL (2006) Food packaging: principles and practice, 2nd edn. Taylor & Francis, New York
Gil L, Barat JM, Escriche I, Garcia-Breijo E, Martinez-Manez R, Soto J (2008) An electronic tongue for fish freshness analysis using a thick-film arry of electrodes. Microchim Acta 163:121–129
Valdés MG, Gonzales ACV, Calzon JAG, Diaz-Garcia ME (2009) Analytical nanotechnology for food analysis. Microchim Acta 166:1–19
Chen N, Chen N, Chen N (2004) Food freshness indicator. US Patent 6,723,285 B2
Huidobro A, Pastor A, Tejada M (2000) Adenosine triphosphate and derivates as freshness indicators of gilthed sea bream. Food Sci Technol Int 7:23–30
Gohil RM (2006) Device for indicating the passage of time an method therefore and articles therewith. Pat. Appl. WO 2006/058228 A1
Hu KH, Loconti JD (1973) Temperature-time integrating indicator. US Patent 3,768,976
Roessler A, Crettenand D, Dossenbach O, Rys P (2003) Electrochemical reduction of indigo in fixed and fluidized beds of graphite granules. J Appl Electrochem 33:901–908
Fanjul-Bolado P, Gonzalez-Garcia MB, Costa-Garcia A (2005) Detection of leucoindigo in alkaline phosphatase and peroxidase based assays using 3-indoxyl phosphate as substrate. Anal Chim Acta 534:231–238
Lewis G (2002) Elapsed time indicator. Pat. Appl. WO 02/46741 A1
Lewis G (2002) Tamper evident packaging. Pat. Appl. WO 02/059010 A1
Biritz LF (1962) Timer device and method for determination. US Patent 3,018,611
Broser W, Kurreck H, Niemeier W (1975) Über paramagnetische Dimerenkomplexe des substituierten Triphenylmetyhlradikals. Tetrahedron 32:1183–1187
Schlenk W, Weickel T, Herzenstein A (1910) Ueber Triphenylmethyl und Analoga des Triphenylmethyls in der Biphenylreihe. Justus Liebigs Ann Chem 372:1–20
Halford JO, Anderson LC (1933) The photochemical production of triphenylmethyl. Proc Natl Acad Sci 19:759–762
Lilly RL, Tummers GLJ, Johnson CD, Davis RD (1980) Elapsed time indicator. US Patent 4,229,813
Diekmann TJ, Bommarito GM (2005) Time or time-temperature indicating articles. US Patent 6,916,116 B2
Komorsky-Lovric S, Mirceski V, Scholz F (1999) Voltammetry of organic microparticles. Microchim Acta 132:67–77
Vuorema A, John P, Keskitalo M, Marken F (2008) Electrochemical determination of plant-derived leuco-indigo after chemical reduction by glucose. J Appl Electrochem 38:1683–1690
Harris JM (1992) Poly(ethylene glycol) chemistry, biotechnical and biomedical applications. Plenum, New York
Clark RJH, Cooksey CJ, Daniels MAM, Withnall R (1993) Indigo, woad, and Tyrian purple: important vat dyes form antiquity to the present. Endeavour 17:191–199
Alcantar NA, Aydil ES, Israelachvili JN (2000) Poly(ethylene glycol)-coated biocompatible surfaces. J Biomed Mater Res A 51:343–351
Fuertges F, Abuchowski A (1990) The clinical efficacy of poly(ethylene glycol) modified proteins. J Control Release 11:139–148
Chen J, Spear SK, Huddleston JG, Rogers RD (2005) Poly(ethylene glycol) and solutions of poly(ethylene glycol) as green reaction media. Green Chem 7:64–82
Li J, Nagai K, Nakagawa T, Wang S (2003) Preparation of poly(ethylene glycol) and cellulose acetate blend membranes, and their gas permeabilities. J Appl Polym Sci 58:1455–1463
Mills A, Tommons C, Bailey RT, Tedford MC, Crilly PC (2008) UV-activated luminescence/colourimetric oxygen indicator. Intl J Photoenergy; open access article ID 547301; doi:10.1155/2008/547301; 6 pp
Weigl BH, Wolfbeis OS (1994) Capillary optical sensors. Anal Chem 66:3323–3327
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wilhelm, S., Wolfbeis, O.S. Opto-chemical micro-capillary clocks. Microchim Acta 171, 211–216 (2010). https://doi.org/10.1007/s00604-010-0456-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-010-0456-4