Abstract
Aims
In previous reports, cardiotocographic (CTG) fetal heart rate (FHR) monitoring has shown only limited benefits in decreasing adverse perinatal outcomes in pregnancies complicated by gestational diabetes mellitus (GDM). The aim of the present study was to evaluate whether an association exists between the recently reported ZigZag pattern (FHR baseline amplitude changes of > 25 bpm with a duration of 2–30 min) and asphyxia-related neonatal outcomes in GDM pregnancies.
Methods
Intrapartal CTGs were recorded in a one-year cohort of 5150 singleton childbirths. The following CTG changes were evaluated: ZigZag pattern, saltatory pattern, late decelerations, episodes of tachycardia and bradycardia, reduced variability, and uterine tachysystole. The cohort was divided into three groups: women with GDM, women with normal oral glucose tolerance test (OGTT), and women with no OGTT performed. Umbilical artery (UA) blood gases, Apgar scores, neonatal respiratory distress, and neonatal encephalopathy were used as outcome variables.
Results
GDM was diagnosed in 624 (12.1%), OGTT was normal in 4115 (79.9%), and OGTT was not performed in 411 (8.0%) women. Hypoxia-related ZigZag patterns (OR 1.94, 95% CI 1.64–2.34) and late decelerations (OR 1.65, 95% CI 1.27–2.13) of FHR, as well as a greater risk of fetal asphyxia (UA pH < 7.10 and/or UA BE < -12.0 meq/L and/or Apgar scores < 7 at 5-min) (OR 6.64, 95% CI 1.84–12.03) were observed in those with GDM compared with those without GDM.
Conclusions
GDM is associated with intrapartal ZigZag pattern and late decelerations, cord blood acidemia and low 5-min Apgar scores at birth indicating increased occurrence of fetal hypoxia in GDM pregnancies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gestational diabetes mellitus (GDM) is the most common medical disorder in pregnancy [1]. It is estimated that about 20 million or 16% of live childbirths worldwide in 2019 were associated with some form of maternal hyperglycemia in pregnancy, of which 84% were due to GDM [2]. GDM is increasing globally mainly as a result of increasing overweight and obesity in women of childbearing age [3]. In Finland, GDM was diagnosed in 19% of all childbirths in 2019 and in 25% of childbirths with maternal age 35 years or older [4].
A linear relationship has been found between fasting, 1-h and 2-h glucose values of the OGTT and perinatal complications [5]. Furthermore, an adequate treatment of GDM can lower the risk of perinatal complications to about the same level as for pregnancies without GDM [3, 5,6,7].
During labor, uterine contractions result in a reduction in uteroplacental perfusion, causing transient fetal and placental hypoxia. A healthy term fetus with a normally developed and functioning placenta is able to adapt to this transient hypoxia without adverse consequences. Women with GDM have higher mean fasting and 2-h post-prandial glucose values compared with mothers without GDM, which may lead to an increase in fetal blood glucose concentration and further adversely affect fetal oxygenation [8].
Cardiotocographic (CTG) monitoring of fetal heart rate (FHR) aims to predict and diagnose fetal hypoxia before fetal compromise occurs [9]. However, previous studies have shown only limited benefit of CTG monitoring in decreasing the risk of adverse fetal and neonatal outcome in GDM pregnancies [10]. Recently, a novel and more detailed analyses of FHR patterns have shown that recognition of the ZigZag pattern (baseline amplitude changes of > 25 bpm with a duration of 2–30 min) may improve the CTG as a screening tool of intrapartum fetal hypoxia in term pregnancies [11,12,13]. The ZigZag pattern precedes late decelerations in the majority of the cases.11 However, it is not known whether the ZigZag pattern of FHR is a sign of imminent fetal compromise in pregnancies complicated by GDM. We hypothesize that GDM may contribute to intrapartum fetal hypoxia and this process can be identified early by the ZigZag pattern in CTG recording during these childbirths.
The aim of the present study was to evaluate whether an association exists between ZigZag pattern of FHR during the last two hours of labor and asphyxia-related neonatal outcomes in GDM pregnancies.
Material and methods
Study population
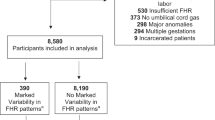
The cohort consisted of 5150 singleton childbirths with continuously monitored CTG tracings during the last two hours of labor with ≥ 33 weeks of gestation at the Maternity Hospital, Helsinki University Hospital (HUS) between January 1 and December 31, 2012. In the Helsinki metropolitan area, the Maternity Hospital took care of low-risk childbirths, excluding, for example, preterm births < 33 weeks of gestation, childbirths of women with severe preeclampsia or type 1 diabetes, and fetuses with severe intrauterine growth restriction. The one-year obstetric cohort is the same as in our recent report on term deliveries, except for the inclusion of preterm (from 33.0 to 36.9 weeks of gestation) deliveries in the present study [11]. All women in the cohort were in the active phase of labor with regular uterine contractions. Preterm pregnancies with < 33 weeks of gestation, non-cephalic presentations, elective cesarean delivery without labor contractions, major congenital malformations, and cases with missing CTG registration or missing UA blood gas results were excluded from the study cohort (Fig. 1). FHR was recorded via a scalp electrode in 91.1% of the cases.
According to the OGTT, the cohort was divided into three groups: GDM, normal OGTT, and no OGTT performed (Fig. 1). The maternal and delivery-related characteristics of the three groups are presented in Table 1.
All women were screened according to the Finnish National Current Care guidelines for gestational diabetes mellitus [4, 14]. The guidelines recommend that all women should be screened for GDM, excluding those with a very low risk (a primipara ≤ 25 years old with a body mass index (BMI) < 25 kg/m2, and without a family history of type 2 diabetes, or a parous woman < 40 years old with a BMI < 25 kg/m2, without a family history of type 2 diabetes and without previous GDM or fetal macrosomia, i.e., birth weight z-score > 2.0 SD-units) [14]. A 2-h OGTT for screening of GDM with 75 g glucose was performed at 24–28 weeks of gestation with the following cut-off values: fasting ≥ 5.3 mmol/l, 1 h value ≥ 10.0 mmol/l, and 2 h value ≥ 8.6 mmol/l. A single abnormal value was diagnostic for GDM [14].
Data sources
The CTGs were recorded using Avalon® FM40 and FM50 (Philips Healthcare, Andover, MA, USA) fetal monitors. All CTG recordings were stored in visual and electronic forms in the Milou® (Medexa, Limhamn, Sweden) CTG database at the Data Analysis and Management Department of HUS. After delivery, CTG recordings were coded and printed on paper for two experienced perinatologists for interpretation. The clinical data were collected from electronic obstetric patient records (Obstetrix®, Obstetrix Medical Group, Englewood, CO, USA). The results of the oral glucose tolerance test (OGTT) were obtained from the HUS Weblab Clinical® laboratory information system.
Evaluation of intrapartum CTG recordings
Two experienced perinatologists (S.S. and K.T.) evaluated the CTG recordings independently and without knowing the maternal, fetal or neonatal data, and perinatal outcomes in order to assess the following CTG changes: ZigZag pattern, saltatory pattern, late decelerations, episodes of bradycardia and tachycardia, reduced FHR variability, and uterine tachysystole. Only concordant CTG changes between the evaluators were used in the analyses. The findings were classified according to the FIGO (The International Federation of Gynecology and Obstetrics) guidelines on intrapartum fetal monitoring with the exception the ZigZag pattern (see below) [15].
Normal baseline FHR was defined as a baseline between 110 and 160 bpm. Normal FHR variability was defined as baseline amplitude changes of 5–25 bpm. The ZigZag FHR pattern was defined as FHR baseline amplitude changes of > 25 bpm with a duration of 2–30 min [11]. The definition of the ZigZag pattern differs from the saltatory pattern in its duration and uniformity of the trace. The saltatory pattern was defined as FHR baseline amplitude changes of > 25 bpm and a duration of > 30 min. Late decelerations were defined as U-shaped decreases of FHR of > 15 bpm occurring late in relation to uterine contractions. In the presence of a tracing without accelerations and with reduced variability, the definition of late decelerations included also those with an amplitude of 10–15 bpm. Tachycardia was defined as a baseline frequency above 160 bpm lasting for more than 10 min. According to the FIGO guideline, FHR values between 100 and 110 bpm may occur in normal fetuses, especially in postdate pregnancies. Thus, in the present study, a bradycardia episode was defined as a baseline frequency below 100 bpm lasting for more than 3 min. The reduced variability was defined as an amplitude below 5 bpm for more than 10 min and that of uterine tachysystole as the occurrence of more than 5 contractions during a 10 min period. Figure 2 shows a CTG recording with ZigZag pattern followed by late decelerations.
Intrapartum CTG recording at 41 + 1 weeks of pregnancy of a 36-year-old nullipara diagnosed with diet-treated GDM. At left, normal baseline FHR frequency (120 bpm/min) and normal variability followed by ZigZag pattern. A 17-min ZigZag episode is followed by repetitive late decelerations. The first stage of labor, cervix dilated 8 cm. No oxytocin augmentation was used. During the ZigZag pattern, no uterine hypertonus was observed, but the change of maternal position and movement of the abdominal toco transducer. A macrosomic male fetus, birth weight 4502 g, birth weight z-score + 2.1 SD, was born vaginally spontaneously 110 min after the occurrence of ZigZag pattern. Umbilical cord blood gas analysis showed acidemia: UA pH 7.05, UA BE -12.4 mmol/L, UA pO2 1.7 kPa. Apgar scores of 6 and 8 at 1 and 5 min, respectively. FHR was recorded via scalp electrode with paper speed 1 cm/min
Maternal and delivery-related variables
The following maternal and delivery-related variables were determined: maternal age, prepregnancy BMI, ethnicity, mode of delivery, type of onset of labor (spontaneous or induction), oxytocin augmentation, gestational age, family history of type 2 diabetes, parity, present or previous GDM, previous fetal macrosomia, preeclampsia, maternal fever ≥ 38.0 °C, and smoking.
Fetal and neonatal variables
The following fetal and neonatal variables were determined: gestational age at delivery, fetal sex, birth weight z-score, umbilical artery (UA) pH, base excess (BE), and pO2, Apgar scores at 1 and 5 min, need for intubation and resuscitation, neonatal intensive care unit (NICU) admission, and neonatal encephalopathy. According to the hospital`s general practice, UA blood was routinely collected from a double-clamped cord for pH and blood gas analyses in all childbirths. Fetal asphyxia was defined as UA pH < 7.10 and/or UA BE < -12.0 meq/L and/or 5-min Apgar scores < 7 [16, 17]. Neonatal respiratory distress was defined as the need for continuous positive airway pressure (CPAP) delivered using a T-piece-based infant resuscitator Neopuff® (Fisher & Paykel Healthcare Limited, Auckland, New Zealand) and/or intubation.
Data analyses
We analyzed continuous variables by Analysis of variance (ANOVA), Kruskal–Wallis test and Mann–Whitney U-test. Pearson`s Chi-square and Fisher's Exact Probability test were used for categorical variables. All tests were two-sided. Values of P < 0.05 were considered statistically significant.
Logistic regression analysis was used to evaluate whether GDM was associated with hypoxia-related CTG changes when the models included parity, type of onset of labor (spontaneous or induction), oxytocin augmentation, obesity (prepregnancy BMI ≥ 30.0 kg/m2), gestational age at delivery (preterm < 37 weeks, term, or postterm ≥ 42 weeks of gestation), maternal age ≥ 35 years, preeclampsia, maternal fever ≥ 38.0 °C, smoking, fetal sex, and fetal macrosomia (birthweight z-score > 2.0 SD-units). The logistic regression analysis was performed by R version 3.6.0 and the odds ratios (OR) and 95% confidence intervals (CI) were estimated by fitting logistic regression models.
Results
Prevalence and interpretation concordance of CTG change
The prevalences of CTG features in women with or without GDM, and in women in whom OGTT was not performed, are presented in Table 2. Of the 5150 childbirths, only CTG changes that were concordant between the two perinatologists were used in the study. Therefore, from the total of 20 562 CTG changes identified of the 5150 CTG recordings, 17 765 concordant CTG changes were used in the final analyses. For the separate CTG changes, the concordance was as follows: for ZigZag pattern 87.2%, for late decelerations 82.1%, for bradycardia episodes 94.0%, for tachycardia episodes 92.1%, for reduced variability 78.3%, and for uterine tachysystole 85.4%. The overall concordance of CTG patterns between the two perinatologists was 86.3%.
Hypoxia-related CTG changes
Fetuses with a ZigZag pattern had a higher risk of intrapartum fetal asphyxia (UA pH < 7.10 and/or UA BE < -12.0 meq/L and/or 5-min Apgar scores < 7) compared with cases without the pattern among women with GDM (OR 1.90, 95% CI 1.31–2.72). The finding was similar for those with late decelerations (OR 1.55, 95% CI 1.19–1.91).
Furthermore, fetuses of GDM women were more likely to have ZigZag pattern (OR 1.94, 95% CI 1.64–2.34) and late decelerations (OR 1.65, 95% CI 1.27–2.13) than fetuses of women with no GDM or in women with no OGTT. Logistic regression analysis revealed that adjustment for maternal and fetal risk factors attenuated the association between GDM and hypoxia-related FHR patterns only marginally (Table 3).
The ZigZag pattern occurred in term pregnancies (≥ 37 weeks of gestation) only. The ZigZag pattern occurred in 304 of the 582 cases (52.2%) during the first stage of labor (cervix dilated < 10 cm) and in 278 cases (47.8%) during the second stage (cervix dilated 10 cm) (P = 0.17 for difference). No differences were observed in the duration of the first and the second stages of labor between the three study groups (Table 1). In the total study population, ZigZag pattern occurred in 91.2% (531/582) of the CTG recordings together with late decelerations. ZigZag pattern preceded late decelerations in 88.7% (471/531) of the cases. A normal FHR preceded the ZigZag pattern in 90.4% (526/582) of the cases, whereas after ZigZag episodes, a normal FHR pattern was observed in 0.9% (5/582) only.
Fetuses of GDM mothers with two abnormal OGTT values had the strongest association with the intrapartal ZigZag pattern (OR 2.27, 95% CI 1.82–2.85). Fasting (OR 1.81, 95% CI 1.36–2.39) hyperglycemia or an abnormal 2-h (OR 1.60, 95% CI 1.19–2.14) OGTT value correlated with the occurrence of ZigZag pattern, whereas no association was found between this FHR pattern and an abnormal 1 h (OR 0.91, CI 95% 0.70–1.17) OGTT value.
Other CTG changes
In all three study groups, bradycardia episodes occurred in approximately half of the cases (Table 2). The majority (78.0%) of the bradycardia episodes appeared during the last 30 min before birth. The presence of episodes with reduced baseline variability was relatively common in all the three study groups (Table 2). The vast majority (92.6%) of the episodes of reduced variability were preceded and followed by normal baseline variability and FHR accelerations.
Furthermore, fetuses of women with GDM had more often tachycardia episodes than women without GDM (OR 1.73, 95% CI 1.39–2.13) (Table 2). However, the vast majority (83.4%) of the tachycardia episodes were preceded and followed by normal frequency of baseline FHR, normal baseline variability, and FHR accelerations. No significant association was found between bradycardia episodes, or reduced variability and GDM (Table 2).
Fetal macrosomia occurred 4.3 times more often in GDM pregnancies compared with pregnancies without GDM (OR 4.31, 95% CI 2.94–6.26). Of the CTG changes, fetal macrosomia was associated with uterine tachysystole (OR 3.13 95% CI 1.84–5.11), but not with ZigZag pattern, late decelerations or episodes of tachycardia. The prevalence of cases with uterine tachysystole was increased in GDM women regardless of the fact that no difference was observed in the use of oxytocin between the three study groups (Table 2).
Fetal and neonatal outcome variables associated with GDM
Table 3 presents the primary fetal and neonatal asphyxia-related outcomes in women with GDM as compared with women without GDM. Newborn infants of mothers with GDM had 6.6-fold risk of intrapartum fetal asphyxia (UA pH < 7.10 and/or UA BE < -12.0 meq/L and/or 5-min Apgar scores < 7) compared with women with no GDM or no OGTT (Table 3). No differences in the occurrence of cord blood acidemia at birth or low 1- or 5 min Apgar scores were found between the cases with normal OGTT and no OGTT performed (Table 2). In women with GDM, newborn infants had 3.6-fold risk of respiratory distress compared with those with normal OGTT and no OGTT performed (Table 3).
Discussion
Principal findings
The present study has three important findings. Firstly, the hypoxia-related ZigZag pattern and late decelerations are significantly more common in GDM pregnancies compared with those without GDM. Secondly, during the last two hours of labor, the occurrence of ZigZag pattern in CTG tracings correlates with intrapartum fetal asphyxia among fetuses of mothers with GDM. Thirdly, GDM is associated with clinical signs of perinatal hypoxia, UA acidemia, low Apgar scores at birth, neonatal respiratory distress, and need for intubation.
Interpretation
The underlying pathophysiology of GDM resulting in fetal hypoxia has been a matter of debate. In GDM pregnancies, mainly placental functional changes occur [18, 19]. However, if impaired glucose metabolism is diagnosed in early pregnancy as a result of more severe form of GDM, structural placental changes have been observed [20, 21]. Furthermore, GDM elicits major changes in the expression of placental genes with a prominent increase in markers and mediators of inflammation [18, 19, 22]. Mild constant hyperglycemia combined with short periods of postprandial hyperglycemia has been shown to increase fetal insulin production.[18] In sheep fetuses, chronic hyperglycemia and secondary hyperinsulinemia increase oxygen consumption and reduce blood oxygen levels [23, 24]. When fetal blood oxygen levels decrease as a result of hyperglycemia, fetal plasma erythropoietin (EPO) levels increase sharply due to increasing fetal EPO synthesis [25]. Consequently, both placental abnormalities and increased oxygen consumption may lead to intrauterine fetal hypoxia [8, 25]. Although the pathophysiology behind fetal hypoxia in hyperglycemic pregnancies has been examined mainly in animal studies and in type 1 diabetes in humans, [23,24,25] elevated markers of fetal hypoxia, such as abnormal cord blood acid–base status and increased cord EPO levels at birth, occur also in GDM pregnancies, [25, 26] suggesting the same underlying pathogenesis. Consequently, throughout the journey of labor, the fetus of a diabetic mother may undergo increased hypoxic stress and therefore, abnormal FHR changes will be seen on the CTG tracing, such as increase in FHR variability, i.e., ZigZag pattern.
In our recent reports, the ZigZag pattern during the last 2 h of labor is associated with fetal intrapartum hypoxia, as indicated by high cord blood EPO levels, cord blood acidemia, and severe neonatal complications [11, 27]. The ZigZag pattern typically precedes late decelerations in CTG tracings, and the fact that normal FHR pattern precedes the ZigZag pattern in the vast majority of the cases, indicates that the ZigZag pattern is an early sign of fetal hypoxia [11, 27]. Furthermore, the ZigZag pattern is independently associated with intrapartum fetal asphyxia even when the pattern occures in the absence of late decelerations [11]. According to our present findings, the ZigZag pattern appears to be an adequate and early indicator of fetal hypoxia also in GDM pregnancies. The increased occurrence of the ZigZag pattern followed by late decelerations in intrapartum CTG tracings is a new observation in GDM pregnancies.
In a 1995 study by Kjos et al., CTG recording was performed on 2134 women with diabetes (1388 women with GDM) [28]. The factors most predictive of cesarean delivery for fetal distress were the presence of late decelerations, nonreactive FHR, and the combination of both nonreactive FHR and FHR decelerations [28]. In a 2011 study by Yli et al., intrapartum CTG monitoring was performed on 413 women with diabetes (307 women with GDM), among whom a preterminal FHR pattern (i.e., FHR pattern with absent FHR baseline variability with or without bradycardia) was more common in fetuses of mothers with type 1 diabetes or GDM than in pregnancies of nondiabetic mothers [29]. However, in the present study, no association was found between episodes of reduced FHR baseline variability or bradycardia and GDM.
Both in pregnancies with and without GDM, bradycardia episodes occurred in approximately 50% of the fetuses, the majority of which took place in the active second stage of labor. This suggests that the bradycardia episodes were caused by fetal head compression, [30] baroreflex activation following increased blood pressure during an overstretched or compressed umbilical cord, [31] or the peripheral chemoreflex triggered by transient periods of acute fall in oxygen tension mediated by frequent uterine contractions [32].
Our observations are in agreement with previous reports that show that women with GDM have a higher risk of low 1- and 5-min Apgar scores [33] and of nonelective operative delivery [34, 35] than women with normal OGTT. Furthermore, in the present and previous studies, GDM was associated with increased risk of neonatal respiratory distress also in term (gestational age ≥ 37 weeks) pregnancies [35, 36]. Furthermore, we found an association between maternal GDM and the occurrence of fetal cord blood acidemia at birth. A similar relation in women with type 1 diabetes or insulin-treated GDM has been observed, [37, 38] but not in women with mild (diet-treated) GDM [34, 35, 39]. Accordingly, several studies conclude that no routine fetal assessment by CTG is needed in diet-controlled term GDM pregnancies without secondary complications [10, 40,41,42].
Strengths and limitations
Strengths of this study are the large number of the study subjects, the use of well-defined criteria of different FHR changes, the high number and quality of the scalp-monitored CTG recordings, and the high concordance between the two experts evaluating the CTG recordings. This is distinctly higher, both in interpretation of a single CTG change as well as in the overall concordance than in previously reported studies [43,44,45]. Although OGTT was not performed in 8.0% of women in the cohort, we find it unlikely that there would be many women with undiagnosed GDM among this group. There was no difference in fetal and neonatal outcome characteristics between women with no GDM and those with no OGTT performed. We, therefore, conclude that very few of the 411 women in whom OGTT was not done, had GDM. Moreover, none of the women were obese (BMI ≥ 30.0 kg/m2) of those with no OGTT performed.
Despite its robust size, our study has some limitations. The first of which is the retrospective study design. Secondly, the evaluation of the CTG recordings was restricted to the last 2 h prior to delivery. Thirdly, fetal macrosomia occurred four times more often in pregnancies of GDM mothers. The absence of data regarding antenatal glycemic controls may contribute to explain fetal hypoxia in macrosomic newborn infants. Finally, it may be difficult to extrapolate our results to the general obstetric population because our study was conducted at a single university center. Therefore, a large population-based multicenter cohort study is needed to confirm these results in the general population.
Conclusions
In conclusion, our findings confirm that maternal GDM may expose the fetus to increased risk of intrapartum hypoxia, which can be detected in CTG recordings as increased ZigZag FHR pattern and other hypoxia-related FHR changes and as asphyxia-related fetal and neonatal outcomes at birth. Understanding this process may improve the clinical decision making on intrapartum CTG recordings in GDM pregnancies and would enable the clinician to plan for possible interventions during labor. We suggest, based on timely recognition of ZigZag and repetitive late decelerations FHR patterns, that continuous CTG recording should be used routinely during labor of GDM women. In order to evaluate the potential long-term effects, future research should include follow-up of offspring with hypoxia-related changes, especially with the ZigZag pattern, in FHR recording during labor in pregnancies of GDM women.
Availability of data and material
The data of this study are available on request from the corresponding author (MT). The data are not publicly available due to privacy and ethical restrictions.
Abbreviations
- BE:
-
Base excess
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CTG:
-
Cardiotocograph
- FHR:
-
Fetal heart rate
- GDM:
-
Gestational diabetes mellitus
- NICU:
-
Neonatal intensive care unit
- OGTT:
-
Oral glucose tolerance test
- OR:
-
Odds ratio
- UA:
-
Umbilical artery
References
Saravanan P (2020) Diabetes in pregnancy working group; maternal medicine clinical study group; Royal College of Obstetricians and Gynaecologists, UK. Gestational diabetes: opportunities for improving maternal and child health. Lancet Diabetes Endocrinol 8(9):793–800. https://doi.org/10.1016/S2213-8587(20)30161-3
International diabetes federation (2019) IDF Diabetes Atlas, (9th edn.) Brussels, Belgium: International diabetes federation, (Pp 53–4). Avalable from: https://www.diabetesatlas.org/upload/resources/material/20200302_133351_IDFATLAS9e-final-web.pdf
Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, Metzger BE et al (2012) The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care 35:780–786. https://doi.org/10.2337/dc11-1790
THL Perinatal statistics: Parturients, deliveries and newborns (2019) Statistical report 48/2020. official statistics of Finland, Perinatal statistics. National institute for health and welfare (THL), Finland, 2020. (Pp 23, 30). Available from: https://www.julkari.fi/bitstream/handle/10024/140702/Tr48_20.pdf?sequence=1&isAllowed=y
Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U (2008) HAPO study cooperative research group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 358:1991–2002. https://doi.org/10.1056/NEJMoa0707943
Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS (2005) Australian carbohydrate intolerance study in pregnant women (ACHOIS) trial group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 352:2477–86. https://doi.org/10.1056/NEJMoa042973
Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B et al (2009) Eunice Kennedy Shriver National Institute of Child Health and human development maternal-fetal medicine units network. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 361:1339–48. https://doi.org/10.1056/NEJMoa0902430
Taricco E, Radaelli T, Rossi G, Nobile de Santis M, Bulfamante G, Avagliano L et al (2009) Effects of gestational diabetes on fetal oxygen and glucose levels in vivo. BJOG 116:1729–35. https://doi.org/10.1111/j.1471-0528.2009.02341.x
Graham EM, Petersen SM, Christo DK, Fox HE (2006) Intrapartum electronic fetal heart rate monitoring and the prevention of perinatal brain injury. Obstet Gynecol 108(3 Pt 1):656–666. https://doi.org/10.1097/01.AOG.0000230533.62760.ef
Landon MB, Vickers S (2002) Fetal surveillance in pregnancy complicated by diabetes mellitus: is it necessary? J Matern Fetal Neonatal Med 12:413–416. https://doi.org/10.1080/jmf.12.6.413.416
Tarvonen M, Hovi P, Sainio S, Vuorela P, Andersson S, Teramo K (2021) Intrapartum zigzag pattern of fetal heart rate is an early sign of fetal hypoxia: a large obstetric retrospective cohort study. Acta Obstet Gynecol Scand 100:252–262. https://doi.org/10.1111/aogs.14007
Gracia-Perez-Bonfils A, Vigneswaran K, Cuadras D, Chandraharan E (2019) Does the saltatory pattern on cardiotocograph (CTG) trace really exist? The ZigZag pattern as an alternative definition and its correlation with perinatal outcomes. J Matern Fetal Neonatal Med 13:1–9. https://doi.org/10.1080/14767058.2019.1686475
Tarvonen M, Hovi P, Sainio S, Vuorela P, Andersson S, Teramo K (2021) Factors associated with intrapartum ZigZag pattern of fetal heart rate: a retrospective one-year cohort study of 5150 singleton childbirths. Eur J Obstet Gynecol Reprod Biol 258:118–125. https://doi.org/10.1016/j.ejogrb.2020.12.056
Working group established by the finnish medical society duodecim, the medical advisory board of the finnish diabetes association, the finnish gynecological association. Current Care Summary: gestational diabetes (2013). Available from: https://www.kaypahoito.fi/en/ccs00047
Ayres-de-Campos D, Spong CY, Chandraharan E (2015) For the FIGO intrapartum fetal monitoring expert consensus panel. FIGO consensus guidelines on intrapartum fetal monitoring: cardiotocography. Int J Gynecol Obstet 131:13–24. https://doi.org/10.1016/j.ijgo.2015.06.020
Yeh P, Emary K, Impey L (2012) The relationship between umbilical cord arterial pH and serious adverse neonatal outcome: analysis of 51,519 consecutive validated samples. BJOG 119:824–831. https://doi.org/10.1111/j.1471-0528.2012.03335.x
Leinonen E, Gissler M, Haataja L, Rahkonen P, Andersson S, Metsäranta M, Rahkonen L (2018) Low Apgar scores at both one and five minutes are associated with long-term neurological morbidity. Acta Paediatr 107:942–951. https://doi.org/10.1111/apa.14234
Desoye G, Hauguel-de Mouzon S (2007) The human placenta in gestational diabetes mellitus. The insulin and cytokine network. Diabetes Care 30(Suppl 2):S120-6. https://doi.org/10.2337/dc07-s203
Radaelli T, Varastehpour A, Catalano P, Hauguel-de MS (2003) Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes 52:2951–2958. https://doi.org/10.2337/diabetes.52.12.2951
Madazli R, Tuten A, Calay Z, Uzun H, Uludag S, Ocak V (2008) The incidence of placental abnormalities, maternal and cord plasma malondialdehyde and vascular endothelial growth factor levels in women with gestational diabetes mellitus and nondiabetic controls. Gynecol Obstet Invest 65:227–232. https://doi.org/10.1159/000113045
Jarmuzek P, Wielgos M, Bomba-Opon D (2015) Placental pathologic changes in gestational diabetes mellitus. Neuro Endocrinol Lett 36:101–105 (PMID: 26071574)
Roberts KA, Riley SC, Reynolds RM, Barr S, Evans M, Statham A et al (2011) Placental structure and inflammation in pregnancies associated with obesity. Placenta 32:247–254. https://doi.org/10.1016/j.placenta.2010.12.023
Philipps AF, Dubin JW, Matty PJ (1982) Arterial hypoxemia and hyperinsulinemia in the chronically hyperglycemic fetal lamb. Pediatr Res 16:653–658. https://doi.org/10.1203/00006450-198208000-00013
Philipps AF, Porte PJ, Stabinsky S, Rosenkrantz TS, Raye JR (1984) Effects of chronic fetal hyperglycemia upon oxygen consumption in the ovine uterus and conceptus. J Clin Inves 74:279–286. https://doi.org/10.1172/JCI111412
Teramo KA, Widness JA (2009) Increased fetal plasma and amniotic fluid erythropoietin concentrations: markers of intrauterine hypoxia. Neonatology 95:105–116. https://doi.org/10.1159/000153094
Beneventi F, Locatelli E, Cavagnoli C, Simonetta M, Lovati E, Lucotti P et al (2014) Effects of uncomplicated vaginal delivery and epidural analgesia on fetal arterial acid-base parameters at birth in gestational diabetes. Diabetes Res Clin Pract 103:444–451. https://doi.org/10.1016/j.diabres.2013.12.019
Tarvonen M, Sainio S, Hämäläinen E, Hiilesmaa V, Andersson S, Teramo K (2020) Saltatory pattern of fetal heart rate during labor is a sign of fetal hypoxia. Neonatology 117:111–117. https://doi.org/10.1159/000504941
Kjos SL, Leung A, Henry OA, Victor MR, Paul RH, Medearis AL (1995) Antepartum surveillance in diabetic pregnancies: predictors of fetal distress in labor. Am J Obstet Gynecol 173:1532–1539. https://doi.org/10.1016/0002-9378(95)90645-2
Yli BM, Källen K, Khoury J, Stray-Pedersen B, Amer-Wåhlin I (2011) Intrapartum cardiotocography (CTG) and ST-analysis of labor in diabetic patients. J Perinat Med 39:457–465. https://doi.org/10.1515/jpm.2011.046
Tranquilli AL (2012) Fetal heart rate in the second stage of labor: recording, reading, interpreting and acting. J Matern Fetal Neonatal Med 25:2551–2554. https://doi.org/10.3109/14767058.2012.718395
Pinas A, Chandraharan E (2016) Continuous cardiotocography during labour: analysis, classification and management. Best Pract Res Clin Obstet Gynaecol 30:33–47. https://doi.org/10.1016/j.bpobgyn.2015.03.022
Lear CA, Galinsky R, Wassink G, Yamaguchi K, Davidson JO, Westgate JA et al (2016) The myths and physiology surrounding intrapartum decelerations: the critical role of the peripheral chemoreflex. J Physiol 594:4711–4725. https://doi.org/10.1113/JP271205
Hildén K, Hanson U, Persson M, Magnuson A, Simmons D, Fadl H (2019) Gestational diabetes and adiposity are independent risk factors for perinatal outcomes: a population based cohort study in Sweden. Diabet Med 36:151–157. https://doi.org/10.1111/dme.13843
El Mallah KO, Narchi H, Kulaylat NA, Shaban MS (1997) Gestational and pre-gestational diabetes: comparison of maternal and fetal characteristics and outcome. Int J Gynaecol Obstet 58:203–209. https://doi.org/10.1016/s0020-7292(97)00084-2
Billionnet C, Mitanchez D, Weill A, Nizard J, Alla F, Hartemann A et al (2017) Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia 60:636–644. https://doi.org/10.1007/s00125-017-4206-6
Kawakita T, Bowers K, Hazrati S, et al (2017) Increased neonatal respiratory morbidity associated with gestational and pregestational diabetes: a retrospective study. Am J Perinatol 34:1160–1168. https://doi.org/10.1055/s-0037-1604414
Klemetti M, Nuutila M, Tikkanen M, Kari MA, Hiilesmaa V, Teramo K (2012) Trends in maternal BMI, glycaemic control and perinatal outcome among type 1 diabetic pregnant women in 1989–2008. Diabetologia 55:2327–2334. https://doi.org/10.1007/s00125-012-2627-9
Castelijn B, Hollander K, Hensbergen JF, IJzerman RG, -van den Valkenburg Berg AW, Twisk J et al (2018) Peripartum fetal distress in diabetic women: a retrospective case-cohort study. BMC Pregnancy Childbirth 14(18):228. https://doi.org/10.1186/s12884-018-1880-4
Suhonen L, Hiilesmaa V, Kaaja R, Teramo K (2008) Detection of pregnancies with high risk of fetal macrosomia among women with gestational diabetes mellitus. Acta Obstet Gynecol Scand 87:940–945. https://doi.org/10.1080/00016340802334377
Salvesen DR, Freeman J, Brudenell JM, Nicolaides KH (1993) Prediction of fetal acidaemia in pregnancies complicated by maternal diabetes mellitus by biophysical profile scoring and fetal heart rate monitoring. Br J Obstet Gynaecol 100:227–33. https://doi.org/10.1111/j.1471-0528.1993.tb15235.x
Jeffery T, Petersen R, Quinlivan J (2016) Does cardiotocography have a role in the antenatal management of pregnancy complicated by gestational diabetes mellitus? Aust N Z J Obstet Gynaecol 56:358–363. https://doi.org/10.1111/ajo.12487
Jabak S, Hameed A (2020) Continuous intrapartum fetal monitoring in gestational diabetes, where is the evidence. J Matern Fetal Neonatal Med 13:1–4. https://doi.org/10.1080/14767058.2020.1849117 (Epup ahead of print)
Schiermeier S, Westhof G, Leven A, Hatzmann H, Reinhard J (2011) Intra- and interobserver variability of intrapartum cardiotocography: a multicenter study comparing the FIGO classification with computer analysis software. Gynecol Obstet Invest 72:169–173. https://doi.org/10.1159/000327133
Rhöse S, Heinis AM, Vandenbussche F, van Drongelen J, van Dillen J (2014) Inter- and intra-observer agreement of non-reassuring cardiotocography analysis and subsequent clinical management. Acta Obstet Gynecol Scand 93:596–602. https://doi.org/10.1111/aogs.12371
Sabiani L, Le Dû R, Loundou A, et al (2015) Intra—and interobserver agreement among obstetric experts in court regarding the review of abnormal fetal heart rate tracings and obstetrical management. Am J Obstet Gynecol 213(856):e1-8. https://doi.org/10.1016/j.ajog.2015.08.066
Acknowledgements
We thank all midwives, doctors, and laboratory workers at the Helsinki University Hospital for assisting in the study. We thank also the all the women who participated in the study.
Funding
Open access funding provided by University of Helsinki including Helsinki University Central Hospital. Mikko Tarvonen has received grants from the Foundation for Pediatric Research (Lastentautien tutkimussäätiö) in Finland and Olga & Vilho Linnamo Foundation. Sture Andersson has received grants from a Special Governmental Subsidy for Clinical Research, Finska Läkaresällskapet, and the Foundation for Pediatric Research in Finland (Lastentautien tutkimussäätiö). The sponsors had no role in the study design; collection, analysis, or interpretation of data, writing of the report, or in the decision to submit the report for publication.
Author information
Authors and Affiliations
Contributions
MT organized data acquisition, collected the data, participated in the study design, and wrote the manuscript. PH analyzed the data and reviewed critically the manuscript. SS evaluated the CTGs and revised the manuscript. PV participated in the study design and reviewed the manuscript critically. SA evaluated the medical records of the newborn infants, participated in the study design, and revised the manuscript. KT evaluated the CTGs, participated in the study design and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial, personal, political, intellectual or religious interests to declare.
Ethical approval
The study was approved by the Institutional Review Board and the Ethics Committee of the Helsinki University Hospital, Finland (no. 361/13/03/03/2010, TMK03§210/15.12.2010).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the topical collection Pregnancy and Diabetes, managed by Antonio Secchi and Marina Scavini.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tarvonen, M., Hovi, P., Sainio, S. et al. Intrapartal cardiotocographic patterns and hypoxia-related perinatal outcomes in pregnancies complicated by gestational diabetes mellitus. Acta Diabetol 58, 1563–1573 (2021). https://doi.org/10.1007/s00592-021-01756-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-021-01756-0