Abstract
Aims
The rate of all-cause mortality is twofold higher in type 2 diabetes than in the general population. Being able to identify patients with the highest risk from the very beginning of the disease would help tackle this burden.
Methods
We tested whether ENFORCE, an established prediction model of all-cause mortality in type 2 diabetes, performs well also in two independent samples of patients with early-stage disease prospectively followed up.
Results
ENFORCE’s survival C-statistic was 0.81 (95%CI: 0.72–0.89) and 0.78 (95%CI: 0.68–0.87) in both samples. Calibration was also good. Very similar results were obtained with RECODe, an alternative prediction model of all-cause mortality in type 2 diabetes.
Conclusions
In conclusion, our data show that two well-established prediction models of all-cause mortality in type 2 diabetes can also be successfully applied in the early stage of the disease, thus becoming powerful tools for educated and timely prevention strategies for high-risk patients.
Similar content being viewed by others
Introduction
The prevalence of type 2 diabetes has shown an alarming increasing trend over the last two decades [1]. In patients with this form of diabetes, the rate of all-cause mortality is twofold higher than in the general population, configuring a major public health problem [2]. Although there is no doubt that type 2 diabetes duration is an independent factor shaping the disease mortality rate [3], it is worth noting that also recently diagnosed patients have an increased risk of death as compared to individuals with no diabetes [4]. To improve the clinical trajectories of patients with the highest risk since the very beginning of the disease, well-performing risk prediction models in this specific clinical subset are needed.
Some years ago, we set up a prediction model of long-term mortality in type 2 diabetes patients [5] that was subsequently refined in a more clinically useful time horizon and named as ENFORCE (EstimatioN oF mORtality risk in type 2 diabetiC patiEnts) [6]. More recently, we have shown that ENFORCE is a flexible model, which may be further improved by the addition of novel biomarkers [7]. Whether ENFORCE performs well also in patients with early-stage type 2 diabetes has never been investigated. The present study was specifically designed to address this issue.
Methods
Discovery set
The Pisa early-stage type 2 diabetes cohort includes all patients referring for the first time to the outpatient diabetes clinic in the department of Internal Medicine from January 2008 to December 2015 and matching these inclusion criteria: age ≥ 30 years and personal history of known type 2 diabetes lasting not more than six months [8]. Diagnosis was confirmed on the basis of the OGTT or HbA1c ≥ 6.5% plus fasting blood glucose ≥ 126 mg/dl. On such basis, 302 patients were identified and included in the prospective observation (further information on baseline measurements and records is given in Supplementary material). For this specific study, 267 patients (88% of the whole sample) having all data on the nine variables comprised in the ENFORCE formula were investigated. The study protocol was approved by the Ethics Committee of the University of Pisa; informed consent was obtained from all participants.
After entering the study, patients were treated according to the good clinical practices recommended by international guidelines and followed a six-monthly/yearly follow-up visits, until death or until December 31, 2018. Mortality from all causes was assessed by verification of the vital state as at December 31, 2018; to this end, we have queried the Italian health card database (http://sistemats1.sanita.finanze.it/wps/portal/), which provides updated information on all current Italian residents.
Validation set
As validation set, all patients with type 2 diabetes diagnosis lasting not more than twelve months (n = 392) were selected among the 3,036 patients comprised in our previous Italian sample where ENFORCE was updated, validated and further improved by additional biomarkers [6, 7] (see details in Supplementary material). There was no overlap between patients from the Pisa early-stage type 2 diabetes cohort (i.e., discovery set) and those from the Pisa Mortality Study (n = 115, 29%) comprised in our Italian sample used for validation.
Statistical methods
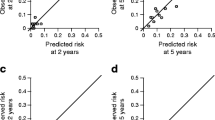
Patient baseline characteristics were reported as frequency (percentage) and mean (SD) or median along with lower and upper quartiles for categorical and continuous variables, respectively. All-cause death incidence rates for 100 person-years were also reported. Overall survival was defined as the time between enrollment and death. For subjects who did not experience the endpoint, survival time was censored at the time of the last available follow-up visit. Discriminatory ability at 6 years was assessed by estimating survival C-statistic, along with 95% CIs derived following perturbation-resampling method [9]. Calibration was also reported as the slope and as the intercept of the regression line between predicted and observed Kaplan–Meier event rates over 6 years by five subgroups at different risk. In an ideal condition, the calibration slope should be 1 and the intercept should be 0, reflecting a perfect agreement between predicted and observed event rates. Calibration was assessed only exploratory due to the low number of events and using the ENFORCE original baseline survival without recalibration assessment.
ENFORCE is a prediction model for 6-year all-cause mortality prediction based on the following nine predictors measured at baseline: age, antihypertensive and insulin therapy, body mass index (BMI), diastolic blood pressure (DBP), low-density lipoprotein (LDL) cholesterol, triglyceride, high-density lipoprotein cholesterol (HDL-C) and albumin/creatinine ratio (ACR) levels [5,6,7]. RECODe (Risk Equations for Complications Of type 2 Diabetes) [10, 11] is a model for 10-year all-cause mortality prediction based on the following fourteen predictors measured at baseline: age, antihypertensive, statins, anticoagulants therapy, systolic blood pressure (SBP), high-density lipoprotein cholesterol (HDL-C) and total cholesterol, albumin/creatinine ratio (ACR) levels, HbA1c, serum creatinine, gender, ethnicity, tobacco smoking and history of cardiovascular disease.
Two-sided P value < 0.05 was considered for statistical significance. All statistical analyses were performed using SAS Software Release 9.4 (SAS Institute, Cary, NC) and the computing environment R (R Development Core Team, version 3.3.2).
Results
The baseline and demographical features, median follow-up and mortality incidence rate of the Pisa early-stage type 2 diabetes patients here investigated are reported in Table 1. A total of 48 deaths occurred during a median follow-up of 4 years, with an incident mortality rate of 3.8 per 100 person-years.
The prediction accuracy by survival C-statistic was 0.81 (95%CI: 0.72–0.89), with good calibration (Supplementary Figure S1A).
Similar results were obtained when the 6-year mortality risk probabilities were computed with RECODe, used to test whether our results were specific for ENFORCE or extendable to other well-performing models (C-statistic = 0.80; 95%CI: 0.72–0.88) with good calibration (Supplementary Figure S1B).
The baseline and demographic features of an independent validation set of 392 subjects with early-stage type 2 diabetes patients are reported in Table 1. In this sample, a total of 68 deaths occurred during a median follow-up of 11 years with an incident mortality rate of 1.7 per 100 person-years. The ENFORCE-derived risk probabilities of 6-year all-cause mortality achieved a C-statistic of 0.78 (95%CI: 0.68–0.87) with good calibration (Supplementary Figure S1C). Similar results were obtained with the RECODe model in terms of both discrimination (Survival C-statistic = 0.78; 95%CI: 0.70–0.87 and calibration (Supplementary Figure S1D).
Conclusions
The present study shows that ENFORCE, an established, inexpensive and easy-to-use prediction model of 6-year all-cause mortality in type 2 diabetes [6], performs well, in terms of both prediction and calibration, also in patients with early-stage disease. Importantly, the transportability of our model in this specific clinical subset was firstly investigated in a training sample [8] and then validated in an independent cohort [6]. Very similar results in terms of performances and validation were obtained with RECODe, an alternative established model for predicting all-cause mortality in type 2 diabetes [10, 11], thus suggesting this is a general property of well-performing models. Notably, ENFORCE and RECODe performed similarly well in the two different study samples which were not superimposable in terms of baseline clinical features and had highly different mortality rates, which indicated that both models are usable in wider clinical contexts of early-stage type 2 diabetes.
Prediction models that function well in early stage of diabetes can help address more effectively the reduction of life expectancy in diabetic patients, one of the major health problems worldwide [1]. Indeed, these tools will allow educated and timely interventions in the highest risk patients, before poor cardio-metabolic control occurs and unescapably influences clinical trajectories for the following decades [12, 13]. While such a precision medicine approach aimed at maximizing effectiveness and minimizing costs is always desirable, it becomes particularly recommendable in these times when many resources of healthcare systems are inevitably oriented at tackling the COVID-19 pandemic.
In conclusion, our data show that two well-established and easy-to-use prediction models of all-cause mortality in type 2 diabetes [5,6,7, 10, 11] can also be applied in the early stage of the disease, thus becoming powerful tools for timely prevention strategies for high-risk patients. The evaluation of their application in wider and more diversified clinical contexts according to best-practice guidelines is advisable to improve the care we provide to diabetic patients.
References
International Diabetes Federation (2017) IDF Diabetes Atlas, 8th edn. International Diabetes Federation. www.diabetesatlas.org, Brussels, Belgium
Rawshani A, Rawshani A, Franzén S et al (2017) Mortality and Cardiovascular Disease in Type 1 and Type 2 Diabetes. N Engl J Med 376(15):1407–1418
Ghouse J, Isaksen JL, Skov MW et al (2020) Effect of diabetes duration on the relationship between glycaemic control and risk of death in older adults with type 2 diabetes. Diabetes Obes Metab 22(2):231–242
Herrington WG, Alegre-Díaz J, Wade R, Gnatiuc L et al (2018) Effect of diabetes duration and glycaemic control on 14-year cause-specific mortality in Mexican adults: a blood-based prospective cohort study. Lancet Diabetes Endocrinology 6(6):455–463
De Cosmo S, Copetti M, Lamacchia O et al (2013) Development and validation of a predicting model of all-cause mortality in patients with type 2 diabetes. Diabetes Care 36(9):2830–2835
Copetti M, Shah H, Fontana A et al (2019) Estimation of Mortality Risk in Type 2 Diabetic Patients (ENFORCE): An Inexpensive and Parsimonious Prediction Model. J Clin Endocrinol Metab 104(10):4900–4908
Scarale MG, Copetti M, Garofolo M et al (2020) The Synergic Association of hs-CRP and Serum Amyloid P Component in Predicting All-Cause Mortality in Patients With Type 2 Diabetes. Diabetes Care 43(5):1025–1032
Biancalana E, Parolini F, Mengozzi A, Solini A (2020) Phenotyping individuals with newly-diagnosed type 2 diabetes at risk for all-cause mortality: a single centre observational, prospective study. Diabetol Metab Syndr 25:12–47
Uno H, Cai T, Pencina MJ, D’Agostino RB, Wei LJ (2011) On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med 30(10):1105–1117
Basu S, Sussman JB, Berkowitz SA et al (2017) Development and validation of Risk Equations for Complications Of type 2 Diabetes (RECODe) using individual participant data from randomised trials. Lancet Diabetes Endocrinology 5(10):788–798
Basu S, Sussman JB, Berkowitz SA et al (2018) Validation of risk equations for complications of type 2 diabetes (RECODe) using individual participant data from diverse longitudinal cohorts in the U.S. Diabetes Care 41(3):586–595
Reddy MA, Zhang E, Natarajan R (2015) Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia 58:443–455
Brownlee M (2005) The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54:1615–1625
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
M.C., V.T. and A.S. participated in the study conception and design. E.B., F.P., M.G. and S.D.C. contributed to data acquisition. M.C. and A.F. performed statistical analyses. M.C., V.T and A.S. participated in data analysis and interpretation. M.C., V.T. and A.S. drafted the manuscript. M.C., S.D.C., O.L., V.T. and A.S. critically reviewed the manuscript. M.C., V.T. and A.S. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that there is no conflict of interest.
Ethics approval
The study protocol was approved by the Ethics Committee of the University of Pisa and of Fondazione IRCCS “Casa Sollievo della Sofferenza”.
Consent to participate
Informed consent was obtained from all participants.
Financial disclosure
No financial disclosures were reported by the authors of this paper.
Additional information
Managed by Antonio Secchi .
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Copetti, M., Biancalana, E., Fontana, A. et al. All-cause mortality prediction models in type 2 diabetes: applicability in the early stage of disease. Acta Diabetol 58, 1425–1428 (2021). https://doi.org/10.1007/s00592-021-01746-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-021-01746-2