Abstract
Aims
Although oxygen is commonly used to treat various medical conditions, it has recently been shown to worsen vascular function (arterial stiffness) in healthy volunteers and even more in patients in whom vascular function might already be impaired. The effects of oxygen on arterial function in patients with type 1 diabetes (T1D) are unknown, although such patients display disturbed vascular function already at rest. Therefore, we tested whether short-term oxygen administration may alter the arterial function in patients with T1D.
Methods
We estimated arterial stiffness by augmentation index (AIx) and the pulse wave velocity equivalent (SI-DVP) in 98 patients with T1D and 49 age- and sex-matched controls at baseline and during hyperoxia by obtaining continuous noninvasive finger pressure waveforms using a recently validated method.
Results
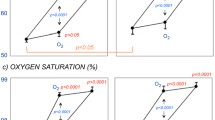
AIx and SI-DVP increased in patients (P < 0.05) but not in controls in response to hyperoxia. The increase in AIx (P = 0.05), systolic (P < 0.05), and diastolic (P < 0.05) blood pressure was higher in the patients than in the controls.
Conclusions
Short-term oxygen administration deteriorates arterial function in patients with T1D compared to non-diabetic control subjects. Since disturbed arterial function plays a major role in the development of diabetic complications, these findings may be of clinical relevance.


Similar content being viewed by others
Abbreviations
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- PP:
-
Pulse pressure
- MAP:
-
Mean arterial pressure
- AIx:
-
Augmentation index
- AIxHR :
-
Heart rate-adjusted augmentation index
- AER:
-
Albumin excretion rate
- T1D:
-
Type 1 diabetes
- SI-DVP:
-
An index of large artery stiffness (SIDVP) derived from the digital volume pulse (DVP)
References
Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C (2010) Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J 31:1865–1871
Chirinos JA, Zambrano JP, Chakko S, Veerani A, Schob A, Willens HJ, Perez G, Mendez AJ (2005) Aortic pressure augmentation predicts adverse cardiovascular events in patients with established coronary artery disease. Hypertension 45:980–985
Gordin D, Wadén J, Forsblom C, Thorn LM, Rosengård-Bärlund M, Heikkilä O, Saraheimo M, Tolonen N, Hietala K, Soro-Paavonen A, Salovaara L, Mäkinen VP, Peltola T, Bernardi L, Groop PH, For the FinnDiane Study Group (2012) Arterial stiffness and vascular complications in patients with type 1 diabetes: The Finnish Diabetic Nephropathy (FinnDiane) Study. Ann Med 44:196–204
Prince CT, Secrest AM, Mackey RH, Arena VC, Kingsley LA, Orchard TJ (2010) Cardiovascular autonomic neuropathy, HDL cholesterol, and smoking correlate with arterial stiffness markers determined 18 years later in type 1 diabetes. Diabetes Care 33:652–657
Giannattasio C, Failla M, Grappiolo A, Gamba PL, Paleari F, Mancia G (2001) Progression of large artery structural and functional alterations in type 1 diabetes. Diabetologia 44:203–208
Waring WS, Thomson AJ, Adwani SH, Rosseel AJ, Potter JF, Webb DJ, Maxwell SR (2003) Cardiovascular effects of acute oxygen administration in healthy adults. J Cardiovasc Pharmacol 42:245–250
Ganz W, Donoso R, Marcus H, Swan HJ (1972) Coronary hemodynamics and myocardial oxygen metabolism during oxygen breathing in patients with and without coronary artery disease. Circulation 45:763–768
Justesen BL, Mistry P, Chaturvedi N, Thom SA, Witt N, Köhler D, Hughes AD, Sjølie AK (2010) Retinal arterioles have impaired reactivity to hyperoxia in type 1 diabetes. Acta Ophthalmol 88:453–457
Williamson JR, Chang K, Frangos M, Hasan KS, Ido Y, Kawamura T, Nyengaard JR, van den Enden M, Kilo C, Tilton RG (1993) Hyperglycemic pseudohypoxia and diabetic complications. Diabetes 42:801–813
Miyata T, de Strihou CY (2010) Diabetic nephropathy: a disorder of oxygen metabolism? Nat Rev Nephrol 6:83–95
Bernardi L, Rosengård-Bärlund M, Sandelin A, Mäkinen VP, Forsblom C, Groop PH, FinnDiane Study Group (2011) Short-term oxygen administration restores blunted baroreflex sensitivity in patients with type 1 diabetes. Diabetologia 54:2164–2173
Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820
Sjöberg F, Singer M (2013) The medical use of oxygen: a time for critical reappraisal. J Intern Med 274:505–528
Rosengård-Bärlund M, Bernardi L, Fagerudd J, Mäntysaari M, Af Björkesten CG, Lindholm H, Forsblom C, Wadén J, Groop PH, FinnDiane Study Group (2009) Early autonomic dysfunction in type 1 diabetes: a reversible disorder? Diabetologia 52:1164–1172
Bernardi L, Gordin D, Rosengård-Bärlund M, Mäkinen VP, Mereu R, DiToro A, Groop PH, On behalf of the FinnDiane Study Group (2014) Arterial function can be obtained by noninvasive finger pressure waveform. Int J Cardiol 175:169–171
Sobol BJ, Wanlass SA, Joseph EB, Azarshahy I (1962) Alteration of coronary blood flow in the dog by inhalation of 100 percent oxygen. Circ Res 11:797–802
Ishikawa K, Lee T, Ganz W (1974) Effect of oxygen on perfusion and metabolism of the ischemic myocardium. J Appl Physiol 36:56–59
Wijesinghe M, Perrin K, Ranchord A, Simmonds M, Weatherall M, Beasley R (2009) Routine use of oxygen in the treatment of myocardial infarction: systematic review. Heart 95:198–202
Roffe C, Ali K, Warusevitane A, Sills S, Pountain S, Allen M, Hodsoll J, Lally F, Jones P, Crome P (2011) The SOS pilot study: a RCT of routine oxygen supplementation early after acute stroke–effect on recovery of neurological function at one week. PLoS One 6:e19113
Kilgannon JH, Jones AE, Shapiro NI, Angelos MG, Milcarek B, Hunter K, Parrillo JE, Trzeciak S, Emergency Medicine Shock Research Network (EMShockNet) Investigators (2010) Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA 303:2165–2171
McEniery CM, Yasmin Hall IR, Qasem A, Wilkinson IB, Cockcroft JR, ACCT Investigators (2005) Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol 46:1753–1756
Boutouyrie P, Bussy C, Hayoz D, Hengstler J, Dartois N, Laloux B, Brunner H, Laurent S (2000) Local pulse pressure and regression of arterial wall hypertrophy during long-term antihypertensive treatment. Circulation 101:2601–2606
Bortolotto LA, Hanon O, Franconi G, Boutouyrie P, Legrain S, Girerd X (1999) The aging process modifies the distensibility of elastic but not muscular arteries. Hypertension 34:889–892
Franklin SS (2008) Beyond blood pressure: arterial stiffness as a new biomarker of cardiovascular disease. J Am Soc Hypertens 2:140–151
Rhee MY, Na SH, Kim YK, Lee MM, Kim HY (2007) Acute effects of cigarette smoking on arterial stiffness and blood pressure in male smokers with hypertension. Am J Hypertens 20:637–641
Lund VE, Kentala E, Scheinin H, Klossner J, Helenius H, Sariola-Heinonen K, Jalonen J (1999) Heart rate variability in healthy volunteers during normobaric and hyperbaric hyperoxia. Acta Physiol Scand 167:29–35
Nichols WW (2005) Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens 18:3S–10S
Thorn CE, Matcher SJ, Meglinski IV, Shore AC (2009) Is mean blood saturation a useful marker of tissue oxygenation? Am J Physiol Heart Circ Physiol 296:H1289–H1295
Ewald U, Tuvemo T, Rooth G (1981) Early reduction of vascular reactivity in diabetic children detected by transcutaneous oxygen electrode. Lancet 1:1287–1288
Haitas B, Barnes A, Shogry ME, Weindling M, Rolfe P, Turner RC (1984) Delayed vascular reactivity to ischemia in diabetic microangiopathy. Diabetes Care 7:47–51
Domínguez C, Ruiz E, Gussinye M, Carrascosa A (1998) Oxidative stress at onset and in early stages of type 1 diabetes in children and adolescents. Diabetes Care 21:1736–1742
Urbina EM, Wadwa RP, Davis C, Snively BM, Dolan LM, Daniels SR, Hamman RF, Dabelea D (2010) Prevalence of increased arterial stiffness in children with T1D mellitus differs by measurement site and sex: the SEARCH for Diabetes in Youth Study. J Pediatr 156:731–737
Gordin D, Forsblom C, Rönnback M, Parkkonen M, Wadén J, Hietala K, Groop PH (2008) Acute hyperglycaemia induces an inflammatory response in young patients with type 1 diabetes. Ann Med 40:627–633
Clanton TL (2007) Hypoxia-induced reactive oxygen species formation in skeletal muscle. J Appl Physiol 102:2379–2388
Thomson AJ, Drummond GB, Waring WS, Webb DJ, Maxwell SR (2006) Effects of short-term isocapnichyperoxia and hypoxia on cardiovascular function. J Appl Physiol 101:809–816
Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H, European Network for Non-invasive Investigation of Large Arteries (2006) Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 27:2588–2605
Acknowledgments
This study was supported by grants from Folkhälsan Research Foundation, Academy of Finland (134379), Helsinki University Central Hospital Research Funds (EVO), the Wilhelm and Else Stockmann Foundation, the Perklén foundation, the Waldemar von Frenckell Foundation, the Liv och Hälsa Foundation, the Finnish Medical Society (Finska Läkaresällskapet), the Diabetes Research Foundation, the Paulo Foundation, the Paavo Nurmi Foundation, The Finnish Medical Foundation, the Biomedicum Helsinki Foundation, the Novo Nordisk Foundation, and the Signe and Ane Gyllenberg Foundation, Helsinki. The authors thank Jaana Tuomikangas, Tuula Soppela, Maikki Parkkonen, and Anna-Reetta Salonen from the Division of Nephrology, Department of Medicine, Helsinki University Central Hospital, and Institute of Genetics, Folkhälsan Research Center, Helsinki, Finland, for excellent technical assistance. D.G., L.B., M.R.-B., and A.N. researched data. D.G., L.B., V.-P.M., and P.-H.G. wrote the manuscript and researched data. D.G., L.B., M.R.-B., V.-P.M., A.S.-P., C.F., A.N., and P.-H.G. reviewed/edited the manuscript. P.-H.G. was responsible for the conception, design, and collection of the study and data, writing and editing of the manuscript, and final approval of the manuscript. P.-H.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest
P.-H.G. received lecture fees from Eli Lilly, Boehringer Ingelheim, Novartis, Genzyme, Merck Sharp & Dohme, and Novo Nordisk and is an advisory board member of Boehringer Ingelheim, Novartis, and Cebix. No other potential conflicts of interest relevant to this article were reported.
Human and Animal Rights disclosure
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Declaration of Helsinki 1975, as revised in 2008.
Informed consent disclosure
Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Managed by Antonio Secchi.
On behalf of the FinnDiane Study Group.
Rights and permissions
About this article
Cite this article
Gordin, D., Bernardi, L., Rosengård-Bärlund, M. et al. Oxygen deteriorates arterial function in type 1 diabetes. Acta Diabetol 53, 349–357 (2016). https://doi.org/10.1007/s00592-015-0775-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-015-0775-3